Markers of undifferentiated state of ESC – what’s inside and what’s outside
….
man's dominion
has broken Nature's social union.
Robert Burns, "To a Mouse, on Turning Her Up in Her Nest with the Plough" (1785)
An almost universally accepted definition of stem cells is: undifferentiated cells that are capable on one hand of renewing their own population and on the other hand of producing differentiated progeny. The former is a basic feature of the undifferentiated state common to stem cells and cancer cells, the latter is a defining ‘stemness’ characteristic. The means by which an undifferentiated cell (cancerous or non-cancerous) maintains its defining properties is based on complex interplay of several mechanisms, employing molecular events internal to the cell as well as signals originating outside the cell. These signals are recognized, amplified and relayed so as to activate the relevant downstream cellular processes that would maintain the undifferentiated state. The exogenous and the endogenous mechanisms responsible for the maintenance of the stemness qualities of the cell are intricately interwoven with one another and susceptible to cross-activation.
The undifferentiated state is peculiar in a variety of manners, as it requires that the cell suppresses possible differentiation pathways, at the same time
keeping them primed and alert so that one or the other could be activated at short notice. It is a delicate balance that may easily be tipped in one
direction or another, using exogenous as well as endogenous means of activation. For example, increasing the expression levels of Oct-4, one of the
essential ‘stemness’ proteins by 50 % in murine embryonic stem cells (mESC) induces their differentiation into extraembryonic endoderm and mesoderm, while
a 50 % decrease in the level of expression of Oct-4 would result in differentiation into trophectoderm [
Markers for pluripotency pertaining to mESC and hESC
MOUSE, n.
An animal which strews its path with fainting women.
Ambrose Bierce. In: The Cynic’s Word Book (1906)
There are several endogenous markers of the undifferentiated state that are common between mESC and hESC. This is only natural, as the initial stages of
embryonic development of all mammals share many common features and the relevant basic molecules exhibit a high degree of homology. One of the hallmarks of
an undifferentiated state both in mice and in men is the expression of the Pou5f1 (POU5F1 for the human homologue) gene, coding for the
transcription factor Oct-4 (Oct-3, Oct-3/4). Virtually all Oct-4 transcripts in mammalian zygotes originate from the oocyte, and maternal and embryonic
transcripts co-exist throughout the early stages of embryonic development [
Another marker typical for the undifferentiated state both in the mouse and the man is Sox2 (SOX2 for the human homologue), a transcription factor
expressed in the ICM of mammal embryos [
Nanog (NANOG for the human homologue) is the third of the basic markers of the undifferentiated state which are common for most mammals. During the
embryonic development the expression of Nanog is detected initially in the morula after the stage of compaction, and, subsequently, in the ICM. After the
implantation, Nanog expression persists only in selected regions of the epiblast and the primordial germ cells. Pluripotent cells of murine and human
origin alike express Nanog and inactivation of the Nanog gene in ESC results in their differentiation along the endodermal lineage [
Markers typical for the undifferentiated state of both murine and human ESC are also alkaline phosphatase, surface antigens TRA1–60 and TRA1–81, and the
transcription factor Foxd3 [
The cells of the inner cell mass of the blastocyst express a panel of surface markers that may be used to distinguish murine ES cells from human ES cells.
For example mESC unlike hESC express the stage-specific embryonic antigen SSEA-1, whereas hESC express SSEA-3 and SSEA-4, which are not expressed in mESC
[
It has been recently proposed that some types of pluripotent ESC may exist in more than one state with regard to their epigenome, their expression profile,
their ability to integrate into foreign cellular environments and certain specificities in the molecular signalling mechanisms responsible for maintaining
the state of pluripotency and the differentiation into different cell types. Specifically, some authors favour the concept that some types of stem cells,
rodent pluripotent stem cells in particular, may exist either in ‘naïve’, or ‘ground’ state or in ‘primed’ (for differentiation) state. The two states are
characteristic for two different periods in early rodent embryo development, naïve mESC being derived from pre-implantation embryos while primed mESC
generally originate from post-implantation embryos. Reversion of primed mESC to naïve mESC is possible via introduction of only one exogenous factor (Klf4)
[
As of now, it is unclear whether the naïve state exists in species different from rodents or whether it is unique to rodent ESC only, albeit recently
porcine ESC have been derived which were reportedly similar in their properties to naïve mESC [
Exogenous factors and signalling cascades functioning in the maintenance of the pluripotent state of mESC and hESC
Two old Bachelors were living in one house;
One caught a Muffin, the other caught a Mouse.
Edward Lear, in: “Laughable Lyrics” (1877)
Maintenance of the undifferentiated state of ESC in vitro is heavily dependent on exogenous factors. These may be secreted by feeder cells (in case ESC are grown on a feeder layer) or may be added in the growth medium as supplements (when ESC are maintained in xeno-free conditions). The exogenous factors can, in general, be viewed as ligands binding to their respective receptors, thereby activating various signalling pathways. As a result, the expression of various target genes is modulated so as to maintain the stemness state or, alternatively, to trigger different prospective routes of differentiation.
The defining features of basic signalling pathways responsible for the maintenance of the undifferentiated state (and respectively for the exit thereof) in mESC and hESC are presented below.
LIF signalling (JAK/STAT pathway)
Historically, the first mESC cells have been grown in medium conditioned by teratocarcinoma cells [
LIF is, in essence, a cytokine of the IL-6 family which exerts its effects by binding to a bipartite membrane receptor complex that consists of the LIF
receptor subunit (Lifr) and the gp130 subunit [
Murine double mutants of the Lifr gene created by targeted gene disruption exhibit severe osteopenia, reduced number of motor neurons and
astrocytes and generally do not survive beyond the neonatal period. Similarly, defects in the human homologue of Lifr (LIFR) result in
Stuve-Wiedemann syndrome type 2, a rare congenital condition transmitted in autosomal recessive manner and characterized by bowing of long bones,
respiratory distress, feeding difficulties, and episodic hyperthermia which usually results in early neonatal death [
Lifr alone has a low binding affinity for LIF, but in its complexed state the binding affinity for the ligand greatly increases. The tyrosine kinase Janus (JAK) is
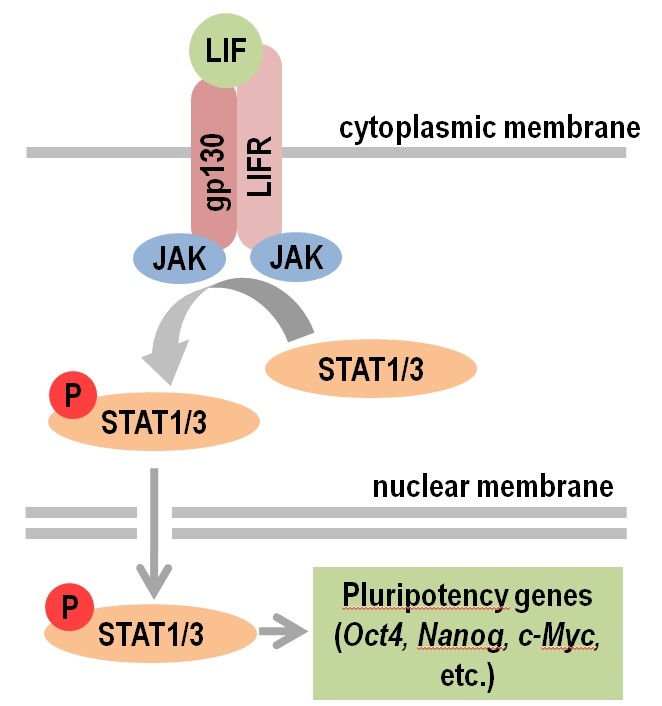
constitutively bound to the cytoplasmic part of the complex Lifr/gp130. JAK is activated by binding of LIF to the receptor complex, which results in
phosphorylation of specific tyrosine residues of gp130 and Lifr. The latter recruits the transcription factors STAT1 (Signal transducer and activator of transcription) and STAT3 [
STAT3 has numerous target genes. Using chromatin immunoprecipitation (ChIP) in 2008 Chen et al. identified 2546 genomic sites for binding of STAT3,
approximately one-third of which (718 sites) were target sites for binding of Oct-4, Sox2 and Nanog [
Among the crucially important target genes activated by STAT3 is the cellular proto-oncogene c-Myc [
The role c-Myc plays in the maintenance of the pluripotent state and the capability for self-renewal of mESC is likely to be implemented via more than one
mechanism. Among these, prominent is the ability of c-Myc to inhibit endodermal differentiation by suppressing its crucial regulator, Gata6 [
STAT3 is a crucial factor in the maintenance of the undifferentiated state of naïve mESC. Its activation by means other than signalling through LIF may
mimic the action of LIF in the maintenance of pluripotency in mESC. In order to maintain the undifferentiated state, however, besides LIF and/or STAT3, the
presence of foetal serum is required, which indicates that there are additional diffusible factors that are needed to maintain self-renewal of mESC in
culture [
TGF-β signalling
As was previously mentioned, LIF alone is sufficient to ensure the maintenance of the pluripotent state of naïve mESC, provided that the growth medium contains foetal calf serum. If the medium is replaced with serum-free medium, however, the cells would spontaneously begin differentiation along the neuronal lineage, regardless of the presence or the absence of LIF. Obviously, the foetal serum contains one or more growth factors acting synergistically with LIF to maintain the pluripotent state
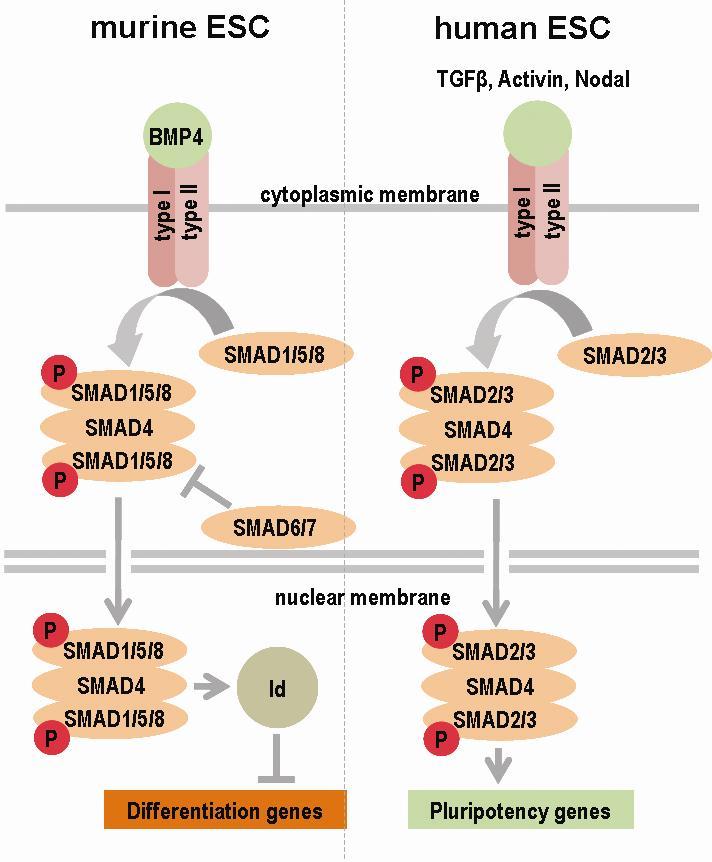
and to preserve the capacity for self-renewal. In 2003, Ying et al. identified the compound in the calf foetal serum partnering LIF in the prevention
of induction of ESC differentiation and the maintenance of the pluripotent state – namely, the bone morphogenic proteins (BMP) 4 [
SMAD proteins are broadly classified into three large categories – R-SMAD (receptor – regulated SMAD); common-mediator SMAD (co-SMAD) and inhibitor SMAD-proteins (I-SMAD). Upon binding of BMP, receptor-regulated SMAD (SMAD1, SMAD5 and SMAD8 in mESC) are phosphorylated by the activated transmembrane tyrosine kinase and form a heterotrimeric
complex with the only co-SMAD protein identified so far in mammals - SMAD4. The heterotrimer enters the nucleus where it functions as a transcription
factor. The process is subject to negative regulation by inhibitor (I)- SMAD (SMAD6 and SMAD7). Namely, I-SMAD repress binding of R-SMAD to co-SMAD (SMAD4)
by competing with SMAD1 for SMAD4, and by stimulating the degradation of the receptor kinases and R-SMAD via the ubiquitin-dependent pathway [
hESC and primed mESC are also sensitive to the presence of BMP4 in the growth medium, but the
mechanism seems to work in exactly the opposite manner – instead of maintaining the pluripotent state, BMP4 stimulates the differentiation of human ESC
into trophectoderm or primitive endoderm [
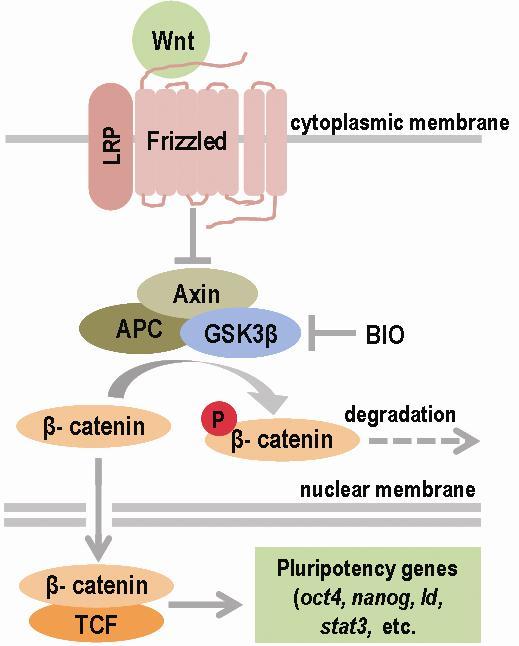
Wnt signalling
The Wnt protein family of ligands are glycoproteins rich in cysteine. Wnt proteins are known to play a role in three signalling pathways, one of which (canonical Wnt pathway) relays signals via β-catenin (Figure 3). The other two pathways, collectively known as non-
canonical Wnt-signalling, transmit signals from the cytoplasmic membrane to the nucleus via other molecules, such as the tyrosine kinase JNK; the small
heterotrimeric G-proteins (small GTP-ases); and also Ca2+ [
The cytoplasmic protein β-catenin plays a basic role in canonic Wnt-signalling. β-catenin has a dual function, linking cadherin receptors to the actin
cytoskeleton in neighbouring cells, thereby constituting an integral part of intercellular contacts, on the one hand and on the other hand acting as an
intracellular messenger [
Signalling pathways mediated by PI3K/Akt
Phosphatidylinositol-3-kinases (PI3К) are a family of proteins with kinase activity, functioning as signal transmitters in cell signalling. PI3К catalyse the phosphorylation of the hydroxyl group in position 3 in the inositol ring of phosphatidylinositol and may be activated by various triggers. Such may be phosphorylation of PI3K by receptor tyrosine kinases
bound to their respective ligands (e.g. growth factors); or binding of regulatory subunits of PI3К class I to phosphorylated receptors. Activation of
PI3K results in generation of second messengers such as phosphatidylinositol-3-phosphate (PI(3)P); phosphatidyl-inositol-(3,4)-bisphosphate (PI(3,4)P 2) and phosphatidyl-inositol-(3,4,5)-trisphosphate (PI(3,4,5)P3). PI(3,4)P2and PI(3,4,5)P3bind
to the pleckstrin homology domain of the serine/threonine kinases of the Akt family (protein kinases B, PKB) and to the
P-domain of the phosphoinositide–dependent protein kinase 1 (PDPK1) and cause translocation of the Akt kinases to the cell membrane and their
subsequent activation [
One of the basic target molecules of Akt is mTOR (mammalian target of rapamycin). mTOR is a serine/threonine kinase which participates in the
regulation of a plethora of cellular processes, among which are cell growth, division, apoptosis, motility, protein synthesis, etc. The activity of
mTOR can be suppressed by adding rapamycin to the growth medium. This results in growth inhibition of ESC (that is, their self-renewal capacity), but
does not trigger differentiation [
Signalling mediated by ERK 1/2
The extracellular-signal-regulated kinases 1 and 2 (ERK1/2) are members of the family of the serine/threonine mitogen-activated protein kinases (MAPK). In
most somatic cells MAPK are involved in the regulation of the progress through the early G1-phase of the cell cycle [
The basic mechanism of activation of MAPK is associated with Ras – cellular proto-oncogene, member of the superfamily of small GTP-ases [
Features in rodent molecular physiology that makes mESC and hESC more unlike each other than expected
…the little mouse, how sagacious an animal it is, which never entrusts its life to one hole only; in as much as, if one hole is blocked up, it seeks
another as a place of refuge.
Titus Maccius Plautus (c. 254–184 BC),
in: Truculentus, Act IV, scene 4
There are only few differences in the exogenous factors required to maintain the undifferentiated state of mESC and hESC. This is not unexpected, to say
the least, as the mouse and the man share between 70 and 90 % similarity in their genomes [
In the light of the current views of existence of two distinct states of pluripotent stem cells, it is believed that primate ESC (hESC included) exhibit
properties more similar to primed (post-implantation) rodent ESC than to naïve (preimplantation) ESC [
There are shared features between naïve mESC and primate ESC, too. For example, both hESC and primate ESC express markers which are not typical of primed
mESC – REX1 being a prime example, and molecules typical for primed ESC such as FGF5 are not found neither in hESC nor in mESC [
Another key difference is in regard to the manner of managing DNA damage in the cells of the early embryo and, respectively, in ESC of murine or human
origin. Since the ability to repair DNA is tightly linked to the ability of the cell to divide, it is obvious that management of DNA damage is crucial in
cell survival and self-renewal. This is of particular importance in the cells of the early embryo, which are expected to divide quickly to form enough
cells so as to lay the progenitors of all cell populations of the adult organism and all DNA-modifying events must be resolved before the cell proceeds to
S phase. Embryonic cells are therefore exquisitely sensitive to the presence of DNA damage [
The cell cycle of ESC of all types is characterized by a shortened G1 phase compared to somatic cells, therefore, all checkpoint mechanisms designed to
prevent damaged cells from entering the cell cycle are relatively relaxed, though to a different degree in different ESC types. Under in vivo and in vitro conditions the source of DNA damage, however, may be quite different. In vivo, the main potential sources of DNA damage in a
dividing embryonic cell are mismatches produced by incorrect template copying (these are usually efficiently resolved by the system of mismatch repair) and
oxidative stress produced by metabolism. Early embryos, however, live in conditions of relative hypoxia and rely on anaerobic glycolysis rather than on
oxidative phosphorylation in order to obtain energy, so the amount of reactive oxygen species (ROS) produced by the cellular metabolism is lower than in
somatic cells. In vitro, however, there might be more DNA damage to deal with, as ESC are often maintained for a long time in culture, and despite
the fact that they age much more slowly than somatic cells, they do experience the cumulative effects of aging. Furthermore, ESC may be treated with
various agents that may cause additional genotoxic stress (e.g. DMSO). In any case, there is a risk of genotoxic damage to embryonic cells, and there is
not much choice, figuratively speaking, on how to proceed with damage resolution. The G1/S phase provides a major checkpoint in eukaryotic cells (also
known as restriction point), and its strictness correlates with the potential risk of letting a cell carrying potentially harmful mutations to produce
progeny [
The situation is somewhat different with human embryos. Human ESC are believed to have preserved their R checkpoint, though its efficiency is lower than in
differentiated cells [
There is also the question of priority of repair in different genomic regions, as it is known that rodent cells
tend to route the NER-associated DNA repair machinery with priority to actively transcribed regions (a.k.a. rodent repairadox [
to proceed with DNA repair first and resort to apoptosis only if this mechanism fails. Coupled with species-specific physiological features, this arrangement seems to work well for both species, albeit in a different manner.
Conclusion
And when they reached their house, they found (besides
their want of Stuffin') The Mouse had fled; - and, previously,had eaten up the Muffin.
Edward Lear, in: “Laughable Lyrics” (1877)
Mice and rats are considered to be close enough to humans to be used as animal models in most research and applications eventually intended to be used in the field of human biomedical science, while being at the same time sufficiently distantly related to primates in aspect of phylogeny so as not to overstep ethical boundaries. This concept satisfies the requirements of many fields in modern biology and medicine, with several exceptions where data from mouse models cannot be translated directly into human research and therapy. Prominent among the latter are some areas of pharmacology, some types of nuclear transactions (e.g. DNA repair) and several aspects of stem cell science. All these can draw a very definite line between the mouse and the man. While basic factors and signalling mechanisms remain the same, they can work in a very different matter in the two species, producing different outcomes. Studying the specific molecular features of both species in the specific context of maintenance of the undifferentiated state of stem cells can provide researchers with the unique opportunity to unravel the complex network of interactions which takes part in the decision of cell fate under different conditions, to glean interesting insights into the parallel evolution of the two species and to observe how different variants of basic cellular processes have been tried and tested in the evolutionary process.
Acknowledgements
This study was supported by grants No. DO02-69 and DO02-180 at the Ministry of Education, Youth and Science of Republic of Bulgaria.References
- Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, et al. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature 1990; 345:686-692.
Reference Link - Wobus AM, Kaomei G, Shan J, Wellner MC, Rohwedel J, Ji Guanju, et al. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular c ardiomyocytes. J Mol Cell Cardiol 1997; 29: 1525-1539.
- Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR et al. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation 2003; 107: 1912-1916
- Sachinidis A, Schwengberg S, Hippler-Altenburg R, Mariappan D, Kamisetti N, Seelig B et al. Identification of small signalling molecules promoting cardiac-specific differentiation of mouse embryonic stem cells. Cell Physiol Biochem 2006; 18: 303-314
- Bader, A, Al-Dubai, H, Weitzer, G Leukemia inhibitory factor modulates cardiogenesis in embryoid bodies in opposite fashions. Circ Res 2000; 86: 787-794
- Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell 2009;4(6): 487-492.
Reference Link - Palmieri SL, Peter W, Hess H, Scholer HR. Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol 1994;166: 259-267
- Zeng F, Schultz RM . RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol 2005;283: 40-57
- Pesce, M., Wang, X., Wolgemuth, D.J., Scholer, H.. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev 1998; 71: 89-98
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000; 24:372-376.
Reference Link - Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005;122: 947-956
- Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol 2005;25: 6031-6046
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998; 95(3): 379-391.
Reference Link - Rem?nyi A, Lins K, Nissen LJ, Reinbold R, Sch?ler HR, Wilmanns M. Crystal structure of a POU/HMG/DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers. Genes Dev 2003; 17(16): 2048-2059.
Reference Link - Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on Sox2 function. Genes Dev 2003; 17: 126-140
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003;113: 631-642
- Picanço-Castro V, Russo-Carbolante E, Covas DT. Forced Expression of Nanog in Human Bone Marrow-Derived Endothelial Cells Activates Other Six Pluripotent Genes. Cellular Reprogramming 2012;14(3): 87-192
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95: 13726-13731
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282: 1145-1147
- Hanna LA, Foreman RK, Tarasenko IA, Kessler DS, Labosky PA. Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev 2002;16(20): 2650-2661.
Reference Link - Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 2009;136: 1063-1069.
Reference Link - Chou YF, Chen HH, Eijpe M, Yabuuchi A, Chenoweth JG, Tesar P, et al. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell 2008; 135(3): 449-461.
Reference Link - Telugu BP, Ezashi T, Sinha S, Alexenko AP, Spate L, Prather RS et al. Leukemia inhibitory factor (LIF)-dependent, pluripotent stem cells established from inner cell mass of porcine embryos. J Biol Chem 2011;286(33): 28948-28953.
Reference Link - De Los Angeles A, Loh YH, Tesar PJ, Daley GQ. Accessing na?ve human pluripotency. Curr Opin Genet Dev 2012;22(3): 272-282.
Reference Link - Tachibana M, Sparman M, Ramsey C, Ma H, Lee H-S,Penedo MCT et al. Generation of chimeric rhesus monkeys. Cell 2012;148: 285-295.
Reference Link - Martin G. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 1981;78(12): 7634-7638
- Wobus AM, Holzhausen H, J?kel P, Sch?neich J. Characterization of a pluripotent stem cell line derived from a mouse embryo. Exp Cell Res. 1984;152(1): 212-219.
Reference Link - Unger C, Skottman H, Blomberg P, Dilber MS, Hovatta O. Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum Mol Genet. 2008;17(R1): R48-R53.
Reference Link - Arabadjiev B, Petkova R, Chakarov S, Momchilova A, Pankov R. Do we need more human embryonic stem cell lines? Biotechnol Biotech Eq 2010; 24(3): 1921-1927.
Reference Link - Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 1988; 336: 684-687.
Reference Link - Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 1988;336: 688-690
Reference Link - Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet 2004;20: 23-32.
Reference Link - Stuve, A., Wiedemann, H.-R. (1971) Congenital bowing of the long bones in two sisters. [Letter] Lancet 1971;298: 495 only
- Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev 1998;12: 2048-2060
- Burdon T, Chambers I, Stracey C, Niwa H, Smith A. Signaling mechanisms regulating self-renewal and differentiation of pluripotent embryonic stem cells. Cells Tissues Organs 1999;165:131-143
- Auernhammer CJ, Melmed S. Leukemia-inhibitory factor - neuroimmune modulator of endocrine function. Endocr Rev 2000;21: 313-345.
Reference Link - Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 2008;133: 1106-1117
- Kidder BL, Yang J, Palmer S. Stat3 and c-Myc genome-wide promoter occupancy in embryonic stem cells. PLoS ONE 2008; 3: e3932
Reference Link - Arceci RJ, King AA, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Molec Cell Biol 1993; 13: 2235-2246
- Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 2005;132(5): 885-896.
Reference Link - Pulverer BJ, Fisher C, Vousden K, Littlewood T, Evan G, Woodgett JR. Site-specific modulation of c-Myc cotransformation by residues phosphorylated in vivo. Oncogene 1994;9(1): 59-70
- . Li Y, Zhang Q, Yin X, Yang W, Du Y, Hou P et al. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res 2011;21(1): 196-204.
Reference Link - Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 2004;10(1): 55-63.
Reference Link - Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J et al. The ground state of embryonic stem cell self-renewal. Nature 2008;453(7194): 519-523.
Reference Link - Smith KN, Singh AM, Dalton S. Myc represses primitive endoderm differentiation in pluripotent stem cells. Cell Stem Cell 2010;7: 343-354
- Wang J, Xie LY, Allan S, Beach D, Hannon GJ. Myc activates telomerase. Genes Dev 1998;12: 1769-1774
- Smith K, Dalton S. Myc transcription factors: key regulators behind establishment and maintenance of pluripotency. Regen Med 2010;5(6): 947-959.
Reference Link - Matsuda T, Nakamura T, Nakao N, Arai T, Katsuki M, Heike T et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J 1999;18: 4261-4269.
Reference Link - Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 2003;115:281-292
- Valdimarsdottir G, Mummery C. Functions of the TGFbeta superfamily in human embryonic stem cells. APMIS 2005;113: 773-789
- Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory SMADs. Mol Biol Cell 2003;14: 2809-2817.
Reference Link - Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol 2003;13: 410-418.
Reference Link - Sharova LV, Sharov AA, Piao Y, Shaik N, Sullivan T, Stewart CL et al. Global gene expression profiling reveals similarities and differences among mouse pluripotent stem cells of different origins and strains. Dev Biol 2007;307(2): 446-59.
Reference Link - Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol 2002;20: 1261-1264
- Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC et al. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells 2005;23: 489-495
- James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 2005;132: 1273-1282
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 2003;5: 367-377.
Reference Link - Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature 2005;434: 843-850
Reference Link - Barker N, Clevers H. Catenins. Wnt signaling and cancer. Bioassays 2000;22(11): 961-965.
Reference Link - Bienz M, Clevers H. Armadillo/beta-catenin signals in the nucleus- proof beyond a reasonable doubt? Nat Cell Biol 2003;5(3): 179-182.
Reference Link - Sato N, Sanjuan IM, Heke M, Uchida M, Naef F, Brivanlou AH. Molecular signature of human embryonic stem cells and its comparison with the mouse. Dev Biol 2003;260: 404-413.
Reference Link - Stephens L, Anderson K, Stokoe D., Erdjument-Bromage H, Painter GF, Holmes AB et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-triphosphate-dependent activation of protein kinase B. Science 1998;279: 710-714
- Takahashi K, Murakami M, Yamanaka S. Role of the phosphoinositide 3-kinase pathway in mouse embryonic stem (ES) cells. Biochem Soc Trans 2005;33: 1522-1525
- Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 2006;25: 2697-2707
- Zhou J, Su P, Wang L, Chen J, Zimmermann M, Genbacev O et al. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc Natl Acad Sci U S A 2009;106(19): 7840-7845.
Reference Link - Lal MA, Bae D, Camilli TC, Patierno SR, Ceryak S. AKT1 mediates bypass of the G1/S checkpoint after genotoxic stress in normal human cells. Cell Cycle 2009;8(10): 1589-1602.
Reference Link - Liu N, Lu M, Tian X, Han Z. Molecular mechanisms involved in self-renewal and pluripotency of embryonic stem cells, J Cell Physiol 2007;211(2): 279-286.
Reference Link - Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 2007;26: 3227-3239
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev 2001; 81: 153-208
- Lee JT Jr, McCubrey JA. The Raf/MEK/ERK signal transduction cascade as a target for chemotherapeutic intervention in leukemia. Leukemia 2002;16: 486-507.
Reference Link - Yoshida-Koide U, Matsuda T, Saikawa K, Nakanuma Y, Yokota T et al. Involvement of Ras in extraembryonic endoderm differentiation of embryonic stem cells. Biochem Biophys Res Commun 2004;313: 475-481.
Reference Link - Burdon T, Stracey C, Chambers I, Nichols J, Smith A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev Biol 1999;210: 30-43.
Reference Link - . Buehr M, Meek S, Blair K, Yan J, Ure J, Silva J et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell 2008;135: 1287-1298.
Reference Link - Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell 2008;135: 1299-1310.
Reference Link - Lanner F, Rossant J. The role of FGF/Erk signaling in pluripotent cells. Development 2010;137(20):3351-3360.
Reference Link - Villegas SN, Canham M, Brickman JM. FGF signalling as a mediator of lineage transitions-evidence from embryonic stem cell differentiation. J Cell Biochem 2010;110(1): 10-20
- Greber B, Coulon P, Zhang M, Moritz S, Frank S, M?ller-Molina AJ et al. FGF signalling inhibits neural induction in human embryonic stem cells. EMBO J 2011;30(24): 4874-4884
- Boguski MS. Comparative genomics: The mouse that roared. Nature 2002;420: 515-516.
Reference Link - Wei CL, Miura T, Robson P, Lim SK, Xu XQ, Lee MY et al. Transcriptome profiling of human and murine ESCs identifies divergent paths required to maintain the stem cell state. Stem Cells 2005;23: 166-185.
Reference Link - Sumi T, Fujimoto Y, Nakatsuji N, Suemori H. STAT3 is dispensable for maintenance of self-renewal in nonhuman primate embryonic stem cells. Stem Cells 2004;22(5): 861-872.
Reference Link - Luo LZ, Gopalakrishna-Pillai S, Nay SL, Park SW, Bates SE, Zeng X et al. DNA Repair in Human Pluripotent Stem Cells Is Distinct from That in Non-Pluripotent Human Cells. PLoS One 2012;7(3): e30541.
Reference Link - Niida H, Nakanishi M. DNA damage checkpoints in mammals. Mutagenesis 2006;21(1): 3-9.
Reference Link - Chakarov S, Roeva I, Russev G. An Experimental Model for Assessment Of Global DNA repair capacity. Biotechnol Biotech Eq 2011;25(3): 2505-2507.
Reference Link - Munroe RJ, Bergstrom RA, Zheng QY, Libby B, Smith R, John SW et al. Mouse mutants from chemically mutagenized embryonic stem cells. Nat Genet 2000;24(3):318-321.
Reference Link - Serrano L, Liang L, Chang Y, Deng L, Maulion C, NguyenS et al. Homologous recombination conserves DNA sequence integrity throughout the cell cycle in embryonic stem cells. Stem Cells Dev 2011;20(2): 363-374.
Reference Link - Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet 2008;9(2): 115-128.
Reference Link - Massague J. G1 cell-cycle control and cancer. Nature 2004;432: 298-306
- Kim Y, Deshpande A, Dai Y, Kim JJ, Lindgren A, Conway A et al. Cyclin-dependent kinase 2-associating protein 1 commits murine embryonic stem cell differentiation through retinoblastoma protein regulation. J Biol Chem 2009; 284(35): 23405-23414.
Reference Link - Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol 2005;7(2): 165-171.
Reference Link - Zhang X, Neganova I, Przyborski S, Yang C, Cooke M, Atkinson SP et al. A role for NANOG in G1 to S transition in human embryonic stem cells through direct binding of CDK6 and CDC25A. Journal of Cell Biology 2009;184: 67-82.
Reference Link - Maynard S, Swistowska AM, Lee JW, Liu Y, Liu ST, Da Cruz AB et al. Human embryonic stem cells have enhanced repair of multiple forms of DNA damage. Stem Cells 2008;26(9): 2266-2274.
Reference Link - Tichy ED, Stambrook PJ. DNA repair in murine embryonic stem cells and differentiated cells. Exp Cell Res 2008;314(9): 1929-1936.
Reference Link - Barta T, Vinarsky V, Holubcova Z, Dolezalova D, Verner J, Pospisilova S et al. Human embryonic stem cells are capable of executing G1/S checkpoint activation. Stem Cells 2010;28(7): 1143-1152
- Neganova I, Vilella F, Atkinson SP, Lloret M, Passos JF, von Zglinicki T et al. An important role for CDK2 in G1 to S checkpoint activation and DNA damage response in human embryonic stem cells. Stem Cells 2011;29(4): 651-659.
Reference Link - Pachkowski BF, Guyton KZ, Sonawane B. DNA repair during in utero development: a review of the current state of knowledge, research needs, and potential application in risk assessment. Mutat Res 2011; 728(1-2): 35-46.
Reference Link - Nygren KG, Finnstrom O, Kallen B, Olausson PO. Population-based Swedish studies of outcomes after in vitro fertilisation. Acta Obstet Gynecol Scand 2007;86: 774-782.
Reference Link - Wennerholm UB, S?derstr?m-Anttila V, Bergh C, Aittom?ki K, Hazekamp J, Nygren KG et al. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod 2009;24(9): 2158-2172
- Check JH, Katsoff B, Wilson C, Choe JK, Brasile D. Pregnancy outcome following fresh vs frozen embryo transfer into gestational carriers using a simplified slow freeze protocol. Clin Exp Obstet Gynecol. 2012;39(1): 23-24
- Hanawalt PC. Preferential repair of damage in actively transcribed DNA sequences in vivo. Genome 1989;31: 605-611.
Reference Link - Nouspikel T, Hanawalt PC. Terminally Differentiated Human Neurons Repair Transcribed Genes but Display Attenuated Global DNA Repair and Modulation of Repair Gene Expression. Molecular and Cellular Biology 2000;20: 1562-1570.
Reference Link - Chakarov S. Russev G. DNA repair and cell differentiation - does getting older means getting wiser as well? Biotechnol Biotech Eq 2010;24(2): 1804-1806
Reference Link - Hyka-Nouspikel N, Lemonidis K, Lu WT, Nouspikel T. Circulating human B lymphocytes are deficient in nucleotide excision repair and accumulate mutations upon proliferation. Blood 2011;117(23): 6277-6286.
Reference Link - Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell 2006;10(1):105-116.
Reference Link