Chemotherapy has been the mainstay of therapy for acute myeloid leukaemia (AML) for over five decades. Despite doses of cytarabine and anthracyclines approaching the limits of haematopoietic tolerance, half of all patients eventually relapse [
The increasing queue of cytotoxic drugs failing to improve clinical outcomes in patients with relapsed and refractory AML indicates the existence of a fundamental mechanism conferring tolerance to genotoxic drugs within leukaemic cells. An attractive hypothesis was that multidrug resistance could be explained by the identification of drug efflux pumps on AML cells. This led to a generation of largely unsuccessful randomised clinical studies combining cytotoxic regimens to inhibitors of multidrug transporters, such as P-glycoprotein [
Thanh-Trang Vo and colleagues from Anthony Letai’s laboratory at Harvard have recently proposed that clinical responses to chemotherapy as well as long-term clinical outcomes may be determined functionally by assessing the capacity of BH3 peptides to induce mitochondrial depolarisation, a method they termed “BH3 profiling” [
The Harvard team applied BH3 profiling to a large cohort of clinically annotated AML samples from the Dana-Farber and Memorial Sloan Kettering. From this work, they concluded that BH3 profiling was a determinant of initial response to induction chemotherapy, relapse after remission, and requirement for allogeneic bone marrow transplantation and that this information could be exploited for the personalization of therapy for AML. From a clinical perspective, how would this information be used? At presentation, most patients fit enough to receive chemotherapy will do so, because of the rapidly progressive nature of the disease. A large proportion of patients with low primed AML still achieve complete remission (CR), so that treatment would unlikely be deterred by this fact. Physicians must be as confident as possible that patients intended for stem cell transplantation are at high risk for relapse and that patients already in a cured state are not unintentionally transplanted. For patients achieving complete remission, BH3 profiling was able to distinguish a sub-group of approximately 20% of patients that were “cured” at that point and did not clinically relapse. All patients in this “cured” group had highly primed AML samples with a >60% response to 0.1μM BimBH3 peptide [
A more intriguing finding in the paper by Vo et al was the suggestion that the dominant pro-survival factor in human AML was Bcl-2, compared to Mcl-1 in normal hematopoietic stem cells (HSCs) [
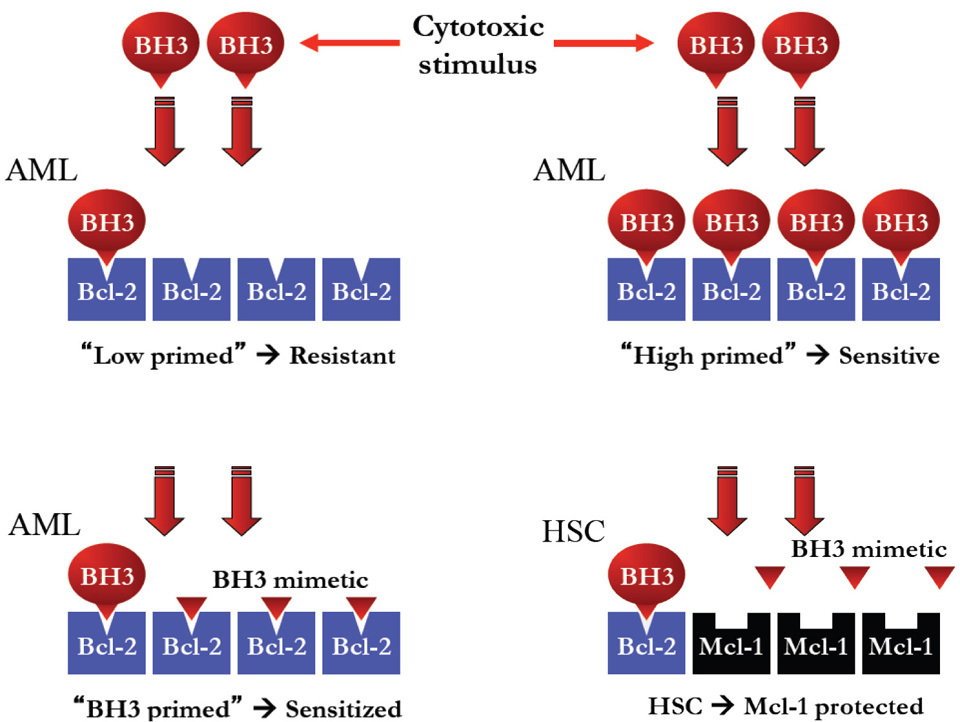
Previously, work by Glaser et al from the Walter and Eliza Hall Institute identified Mcl-1 as a critical survival factor in AML [
Next generation sequencing has shown that hundreds of coding mutations potentially exist within each AML genome [
Acknowledgments
We wish to acknowledge funding support received from the Leukaemia Foundation of Australia, the Victorian Cancer Agency and the National Health and Medical Research Council of Australia.
References
- Fernandez H, Sun Z, Yao X, Litzow M, Luger S, Paietta E, et al. Anthracycline dose intensification in acute myeloid leukemia. The New England Journal of Medicine 2009; 361: 1249-1259.
Reference Link - Iland H, Bradstock K, Supple S, Catalano A, Collins M, Hertzberg M, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood 2012; 120: 1570-1580.
Reference Link - Levis M, Ravandi F, Wang E, Baer M, Perl A, Coutre S, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood 2011; 117: 3294-3301.
Reference Link - Smith C, Wang Q, Chin C-S, Salerno S, Damon L,1 Levis M, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 2012; 485: 260-263.
- Man C, Fung T, Ho C, Han H, Chow H, Ma A, et al. Sorafenib treatment of FLT3-ITD+ acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent non-responsiveness associated with a D835 mutation. Blood 2012; 119: 5133-5143.
Reference Link - Becton D, Dahl G, Ravindranath Y, Chang M, Behm F, Raimondi S, et al. Randomized use of cyclosporin A (CsA) to modulate P-glycoprotein in children with AML in remission: Pediatric Oncology Group Study 9421. Blood 2006; 107: 1315-1324.
Reference Link - van der Holt B, Löwenberg B, Burnett A, Knauf W, Shepherd J, Piccaluga P, Ossenkoppele G, et al. The value of the MDR1 reversal agent PSC-833 in addition to daunorubicin and cytarabine in the treatment of elderly patients with previously untreated acute myeloid leukemia (AML), in relation to MDR1 status at diagnosis. Blood 2005; 106: 2646-2654.
- Li R, Sutphin PD, Schwartz D, Matas D, Almog N, Wolkowicz R et al. Mutant p53 protein expression interferes with p53-independent apoptotic pathways. Oncogene 1998; 16: 3269-3277.
Reference Link - Stirewalt DL, Kopecky KJ, Meshinchi S, Appelbaum FR, Slovak ML, Willman CL, et al. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood 2001; 97: 3589-3595.
Reference Link - Fenaux P, Jonveaux P, Quiquandon I, Laï JL, Pignon JM, Loucheux-Lefebvre MH, et al. P53 gene mutations in acute myeloid leukemia with 17p monosomy. Blood 1991; 78: 1652-1657.
- Haferlach C, Dicker F, Herholz H, Schnittger S, Kern W, Haferlach T. Mutations of the TP53 gene in acute myeloid leukemia are strongly associated with a complex aberrant karyotype. Leukemia 2008; 22: 1539-1541.
Reference Link - Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. The EMBO Journal 2011; 30: 3667-3683.
Reference Link - Cheng E, Wei M, Weiler S, Flavell R, Mak T, Lindsten T, et al. BCL-2, BCL-XL Sequester BH3 Domain-Only Molecules Preventing BAX-and BAK-Mediated Mitochondrial Apoptosis. Molecular Cell 2001; 8: 705-711.
Reference Link - Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner M, et al. p53-and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science Signalling 2003; 302: 1036.
- Schmitt CA, Lowe SW. Bcl-2 Mediates Chemoresistance in Matched Pairs of Primary Eμ-myc Lymphomas in Vivo. Blood Cells, Molecules, and Diseases 2001; 27: 206-216.
Reference Link - Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 2005; 17: 393-403.
Reference Link - Vo, T, Ryan J, Carrasco R, Neuberg D, Rossi D, Stone RM, et al. Relative Mitochondrial Priming of Myeloblasts and Normal HSCs Determines Chemotherapeutic Success in AML. Cell 2012; 151: 344-355.
Reference Link - Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science Signalling 2001; 292: 727-730.
- Mérino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, et al. Bcl-2, Bcl-xL, and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 2012; 119: 5807-5816.
- Oltersdorf T, Steven W. Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435: 677-681.
- Mason K, Vandenberga CJ, Scotta CL, Wei AH, Corya S, Huanga D, et al. In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proceedings of the National Academy of Sciences 2008; 105: 17961-17966.
Reference Link - Reed, J. C. Bcl-2 family proteins: regulators of apoptosis and chemoresistance in hematologic malignancies. Semin Hematol 1997; 34: 9-19.
- Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie D, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes & Development 2012; 26: 120-125.
Reference Link - Lee EF, Czabotar P, van Delft MF, Michalak EM, Boyle MJ, Willis SN, et al. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. The Journal of Cell Biology 2008; 180: 341-355.
Reference Link - Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990; 348: 334-336.
Reference Link - Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proceedings of the National Academy of Sciences 1997; 94: 3668-3672.
Reference Link - Doi K, Li R, Sung SS, Wu H, Liu Y, Manieri W, et al. Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. Journal of Biological Chemistry 2012; 287: 10224-10235.
Reference Link - Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 2012; 481: 506-510.
Reference Link - Kohl TM, Hellinger C, Ahmed F, Buske C, Hiddemann W, Bohlander SK, et al. BH3 mimetic ABT-737 neutralizes resistance to FLT3 inhibitor treatment mediated by FLT3-independent expression of BCL2 in primary AML blasts. Leukemia 2007; 21: 1763-1772.
Reference Link - Kojima K, Konopleva M, Samudio IJ, Schober WD, Bornmann WG, Andreeff M, et al. Concomitant inhibition of MDM2 and Bcl-2 protein function synergistically induce mitochondrial apoptosis in AML. Cell Cycle 2006; 5: 2778-2786.
Reference Link - Tsao T, Shi Y, Kornblau S, Lu H, Konoplev S, Antony A, et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Annals of Hematology 2012; 91: 1-10.
Reference Link - Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, et al. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia 2011; 26: 778-787.
Reference Link