Introduction
Skeletal muscles support physical activity and generate large energy with muscle contraction. In addition, these muscles release various metabolic factors such as lactate, amino acids, and ammonia into circulation in response to physiological changes. Growing evidence has shown that muscle cells secrete also bioactive proteins, which have regulatory role in the muscles and other organs via endocrine, autocrine, or paracrine actions; this is the so-called myokine theory [
Adequate regular exercise has numerous health benefits. In the last few decades, epidemiological studies have shown that dietary–exercise regimen reduces the risk of various common diseases such as type 2 diabetes, cardiovascular disease, and carcinogenesis. In addition, regular exercise improves the prognosis of existing diseases, including diabetes, ischemic heart disease, heart failure, and chronic obstructive pulmonary disease. Accumulating evidence has demonstrated the mechanisms underlying the benefits of acute and regular exercise. A single bout of exercise drastically changes various physiological parameters such as hormone production, blood flow, and the activity of the nervous and immune system, in addition to altering the expression/activity of certain genes and proteins in the skeletal muscle. Further, regular exercise adaptively improves normal bodily functions including energy metabolism, muscle strength, brain-nervous system, endocrine system, and immune function, even in resting state, and the expression/activity of several key proteins in the skeletal muscle is involved in the development of this adaptation. The bioactive proteins secreted from the muscle would contribute in promoting health benefits along with maintaining physiological homeostasis and sports performance during exercise.
Metabolic and immune functions of muscle-secreted proteins
Previously, several proteins that are secreted from muscle cells into the extracellular environment in response to exercise have been reported. Many of them were suggested to be involved in the regulation of metabolic function in skeletal muscle itself and also in other metabolic organs. Interleukin (IL) -6 is a well-known secretory protein that is transiently elevated in muscles following a single bout of exercise [
In addition to IL-6, other muscle-secreted proteins such as brain-derived neurotrophic factor, fibroblast growth factor 21, IL-15, and myonectin have been shown to be produced in skeletal muscle in response to acute or chronic exercise, and have been suggested to increase fat oxidation or glucose uptake in skeletal muscles [
Anti-inflammation is another function suggested for muscle-secreted proteins, and muscle-derived IL-6 likely contributes to reduce inflammation when in circulation [
Myogenic function of muscle-secreted protein
Several proteins contribute to muscle hypertrophy via autocrine or paracrine effects. Insulin growth factor-1 (IGF-1) is known as a major hypertrophic inducer. It has been considered for long time that IGF-1 is generated by stimulating growth hormone in liver and secreted into circulation [
Myostatin, a member of the transforming growth factor-β family, is a negative regulator of muscle hypertrophy. Originally, although myostatin is recognized to affect to the intracellular signaling such as calcineurin pathway [
IL-15 is also known as a muscle-secreted protein which can regulate muscle mass via inhibiting protein degradation and accelerating differentiation [
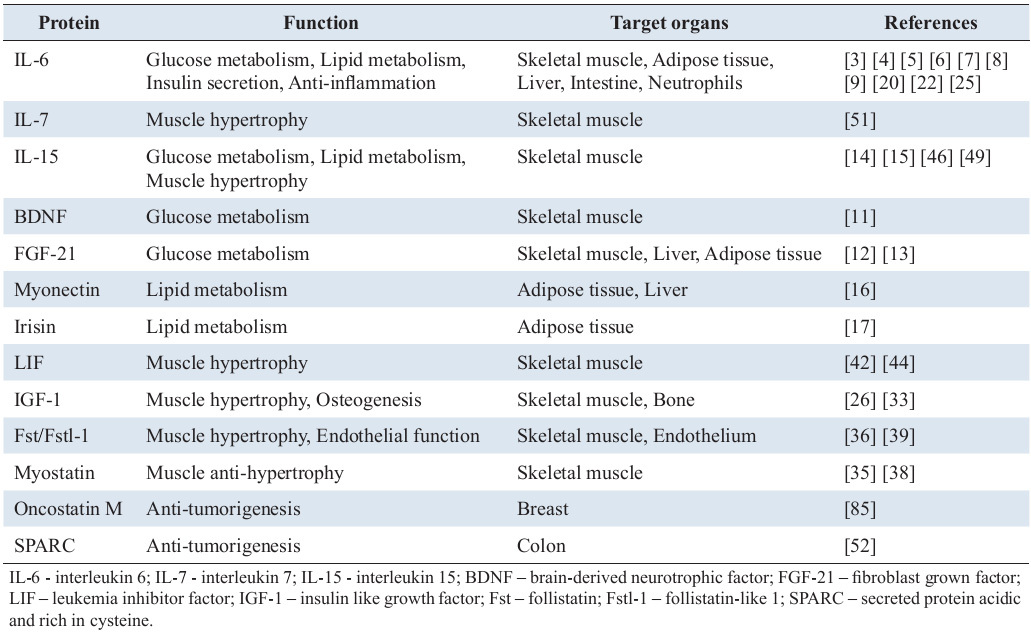
SPARC is a cancer preventive protein secreted by skeletal muscle
We recently tried to identify novel muscle-derived proteins that are secreted into the general circulation. The transcriptome of muscle tissue in sedentary and exercised young and old mice were compared. In total, 381 genes in gastrocnemius muscle were up-regulated in mice that exercised for 4 weeks compared with sedentary mice; on the other hand, 100 genes were downregulated in 24-month-old sedentary mice compared with 3-month-old sedentary mice [
![Figure 1. A single bout of exercise increases circulating levels of SPARC in humans. Time course of serum SPARC level after steady-state cycling at 70% maximal oxygen uptake (VO2max) for 30 min (n = 10). *P < 0.05 versus resting state (Rest). *Significant difference between resting state (Rest) and immediately after exercise (Post-Ex) are depicted by (*) for P < 0.05. Results are shown as mean ± standard error. Data from Aoi et al. [52]. Figure 1](https://biodiscovery.pensoft.net/showimg/oo_85788.jpg)
A number of epidemiological studies have focused on the relationships between the average individual’s level of physical activity and the incidence of cancer in Europe, the United States, and Japan. The general consensus among the authors of these studies is that physical activity can prevent cancer in the colon, breast, uterus, pancreas, and lungs [
SPARC is a matricellular protein that is primarily involved in development, remodeling, and tissue repair through modulation of cell-cell and cell-matrix interactions [
![Figure 2. SPARC prevents tumorigenesis in colon. The numbers of aberrant crypt foci (ACF) on the mucosal surface of the colon were counted under a light microscope. In wild-type mice, regular low-intensity exercise significantly reduced the number of ACF in the colons of AOM-treated mice compared to sedentary mice. In contrast, more ACF were formed in AOM-treated SPARC-null mice than in wild-type mice, and exercise did not have an inhibitory effect. Results are shown as mean ± standard error (n = 10–12). AOM, AOM-treated sedentary mice; AOM-Ex, AOM-treated exercised mice. *P < 0.05 and ** represents P < 0.01. Data from Aoi et al. [52]. Figure 2](https://biodiscovery.pensoft.net/showimg/oo_85789.jpg)
A cause of ACF formation is dysregulation of apoptosis [
Prospective
Many studies have suggested that there are muscle-secreted proteins yet to be identified. For example, a bioinformatics study showed that the secretome of human muscle cells includes more than 300 proteins [
It is well-known from previous studies that exercise releases various metabolic factors from skeletal muscle into circulation. For example, lactate is generated from carbohydrates via glycolytic metabolism and the amount is based on the intensity of exercise. After its release into blood, lactate is carried to other tissues and is utilized as a substrate of aerobic metabolism or gluconeogenesis. Recently, studies into further functions of lactate have shown that exogenous lactate mediates insulin-induced anti-lipolytic effect via G-protein coupled receptor GPR81 located on plasma membrane [
Conclusion
Skeletal muscle secretes several bioactive proteins from within the cell into extracellular fluid. The secretion of several proteins, whose levels increase in response to exercise, can mediate exercise-induced benefits such as metabolic improvement, anti-inflammation, and muscle hypertrophy. We recently found a novel muscle-secreted protein SPARC which may be fundamental for the colon cancer prevention mechanism of regular exercise, demonstrated by various epidemiological studies. Many other proteins, along with c-miRNAs in exosome and metabolites, secreted from muscle have yet to be identified. In the future, the presence and beneficial function of more unknown bioactive factors are expected to be discovered, which strengthens the development of sports science.
Acknowledgments
This work was supported by Grants-in-Aid from the Japan Society for the Promotion of Science (23700776W.A.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P et al. Searching for the exercise factor: is IL-6 a candidate. J Muscle Res Cell Motil 2005; 24: 113–119.
Reference Link - Pedersen BK, Fischer CP. Beneficial health effects of exercise--the role of IL-6 as a myokine. Trends Pharmacol Sci 2007; 28: 152–156.
Reference Link - Penkowa M, Keller C, Keller P, Jauffred S, Pedersen BK. Immunohistochemical detection of interleukin-6 in human skeletal muscle fibers following exercise. FASEB J 2003; 17: 2166–2168.
- Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exercise Immunol Rev 2006; 12: 6–33.
- Benrick A, Wallenius V, Asterholm IW. Interleukin-6 mediates exercise-induced increase in insulin sensitivity in mice. Exp Physiol 2012; 97: 1224–1235.
Reference Link - van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab 2003; 88: 3005–3010.
Reference Link - Petersen EW, Carey AL, Sacchetti M, Steinberg GR, Macaulay SL, Febbraio MA et al. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro: evidence that IL-6 acts independently of lipolytic hormones. Am J Physiol Endocrinol Metab 2005; 288: E155–E162.
Reference Link - Lyngso D, Simonsen L, Bulow J. Metabolic effects of interleukin-6 in human splanchnic and adipose tissue. J Physiol 2002; 543: 379–386.
Reference Link - Lienenlüke B, Christ B. Impact of interleukin-6 on the glucose metabolic capacity in rat liver. Histochem Cell Biol 2007; 128: 371–377.
Reference Link - Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 2011; 17: 1481–1489.
Reference Link - Matthews VB, Aström MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009; 52: 1409–1418.
- Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev 2011; 27: 286–297.
Reference Link - Cuevas-Ramos D, Almeda-Valdés P, Meza-Arana CE, Brito-Córdova G, Gómez-Pérez FJ, Mehta R et al. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One 2012; 7: e38022.
- Busquets S, Figueras M, Almendro V, López-Soriano FJ, Argilés JM. Interleukin-15 increases glucose uptake in skeletal muscle. An antidiabetogenic effect of the cytokine. Biochim Biophys Acta 2006; 1760: 1613–1617.
- Tamura Y, Watanabe K, Kantani T, Hayashi J, Ishida N, Kaneki M. Upregulation of circulating IL-15 by treadmill running in healthy individuals: is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr J 2011; 58: 211–215.
Reference Link - Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 2012; 287: 11968–11980.
Reference Link - Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463–468.
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 2012; 16: 1879–1886.
Reference Link - Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C et al. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 2003; 52: 2874–2881.
Reference Link - Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 2003; 285: E433–E437.
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990; 265: 621–636.
- Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen, BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J 2003; 17: 884–886.
- Kadoglou NP, Perrea D, Iliadis F, Angelopoulou N, Liapis C, Alevizos M. Exercise reduces resistin and inflammatory cytokines in patients with type 2 diabetes. Diabetes Care 2007; 30: 719–721.
Reference Link - Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB et al. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc 2008; 56: 2045–2052.
Reference Link - LeRoith D, Roberts CT Jr. Insulin-like growth factor I (IGF-I): a molecular basis for endocrine versus local action?. Mol Cell Endocrinol 1991; 77: C57–C61.
Reference Link - Adams GR. Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol 2002; 93: 1159–1167.
- Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 1997; 272: 6653–6662.
Reference Link - Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y et al. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 2005; 280: 2737–2744.
Reference Link - Semsarian C, Wu MJ, Ju YK, Marciniec T, Yeoh T, Allen DG et al. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature 1999; 400: 576–581.
Reference Link - Sakuma K, Nishikawa J, Nakao R, Watanabe K, Totsuka T, Nakano H et al. Calcineurin is a potent regulator for skeletal muscle regeneration by association with NFATc1 and GATA-2. Acta Neuropathol 2003; 105: 271–280.
- Chew SL, Lavender P, Clark AJ, Ross RJ. An alternatively spliced human insulin-like growth factor-I transcript with hepatic tissue expression that diverts away from the mitogenic IBE1 peptide. Endocrinology 1995; 136: 1939–1944.
Reference Link - Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol 2003; 547: 247–254.
Reference Link - Yu Y, Mu J, Fan Z, Lei G, Yan M, Wang S et al. Insulin-like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem Cell Biol 2012; 137: 513–525
Reference Link - Sakuma K, Yamaguchi A. The functional role of calcineurin in hypertrophy, regeneration, and disorders of skeletal muscle. J Biomed Biotechnol 2010; 2010: 721219.
- Hittel DS, Berggren JR, Shearer J, Boyle K, Houmard JA. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 2009; 58: 30–38.
Reference Link - Kocamiş H, Gulmez N, Aslan S, Nazli M. Follistatin alters myostatin gene expression in C2C12 muscle cells. Acta Vet Hung 2004; 52: 135–141.
Reference Link - Diel P, Schiffer T, Geisler S, Hertrampf T, Mosler S, Schulz S et al. Analysis of the effects of androgens and training on myostatin propeptide and follistatin concentrations in blood and skeletal muscle using highly sensitive immuno PCR. Mol Cell Endocrinol 2010; 330: 1–9.
Reference Link - Willoughby DS. Effects of an alleged myostatin-binding supplement and heavy resistance training on serum myostatin, muscle strength and mass, and body composition. Int J Sport Nutr Exerc Metab 2004; 14: 461–472.
- Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C Izumiya Y et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem 2008; 283: 32802–32811.
- Diao Y, Wang X, Wu Z. SOCS1, SOCS3, and PIAS1 promote myogenic differentiation by inhibiting the leukemia inhibitory factor-induced JAK1/STAT1/STAT3 pathway. Mol Cell Biol 2009; 29: 5084–5093.
Reference Link - Sun L, Ma K, Wang H, Xiao F, Gao Y, Zhang W, et al. JAK1-STAT1-STAT3, a key pathway promoting proliferation and preventing premature differentiation of myoblasts. J Cell Biol 2007; 179: 129–138.
Reference Link - Hunt LC, Tudor EM, White JD. Leukemia inhibitory factor-dependent increase in myoblast cell number is associated with phosphatidylinositol 3-kinase-mediated inhibition of apoptosis and not mitosis. Exp Cell Res 2010; 316: 1002–1009.
Reference Link - Broholm C, Pedersen BK. Leukaemia inhibitory factor--an exercise-induced myokine. Exerc Immunol Rev 2010; 16: 77–85.
- Broholm C, Mortensen OH, Nielsen S, Akerstrom T, Zankari A, Dahl B et al. Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J Physiol 2008; 586: 2195–2201.
Reference Link - Carbó N, López-Soriano J, Costelli P, Busquets S, Alvarez B, Baccino FM et al. Interleukin-15 antagonizes muscle protein waste in tumour-bearing rats. Brit J Cancer 2000; 83: 526–531.
- Quinn LS, Anderson BG, Drivdah RH, Alvarez B, Argilés JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res 2002; 280: 55–63.
Reference Link - Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T et al. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol 2007; 584: 305–312.
Reference Link - Nieman DC, Davis JM, Brown VA, Henson DA, Dumke CL, Utter AC et al. Influence of carbohydrate ingestion on immune changes after 2 h of intensive resistance training. J Appl Physiol 2004; 96: 1292–1298.
Reference Link - Quinn LS, Anderson BG, Strait-Bodey L, Wolden-Hanson T. Serum and muscle interleukin-15 levels decrease in aging mice: correlation with declines in soluble interleukin-15 receptor alpha expression. Exp Gerontol 2010; 45: 106–112.
Reference Link - Pistilli EE, Siu PM, Alway SE. Interleukin-15 responses to aging and unloading-induced skeletal muscle atrophy. Am J Physiol Cell Physiol 2007; 292: C1298–C1304.
Reference Link - Haugen F, Norheim F, Lian H, Wensaas AJ, Dueland S, Berg O et al. IL-7 is expressed and secreted by human skeletal muscle cells. Am J Physiol Cell Physiol 2010; 298: C807–C816.
Reference Link - Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 2012; e-pub ahead of print 16 November 2012.
- Garabrant DH, Peters JM, Mack TM, Bernstein L. Job activity and colon cancer risk. Am J Epidemiol 1984; 119: 1005–1014.
- Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S et al. Physical activity and risk of colorectal cancer in Japanese men and women: the Japan Public Health Center-based prospective study. Cancer Causes Control 2007; 18: 199–209.
Reference Link - Mai PL, Sullivan-Halley J, Ursin G, Stram DO, Deapen D, Villaluna D et al. Physical activity and colon cancer risk among women in the California Teachers Study. Cancer Epidemiol Biomarkers Prev 2007; 16: 517–525.
Reference Link - Vena JE, Graham S, Zielezny M, Brasure J, Swanson MK. Occupational exercise and risk of cancer. Am J Clin Nutr 1987; 45: 318–327.
- Zheng W, Shu XO, McLaughlin JK, Chow WH, Gao YT, Blot WJ. Occupational physical activity and the incidence of cancer of the breast, corpus uteri, and ovary in Shanghai. Cancer 1993; 71: 3620–3624.
Reference Link - Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. J Am Med Assoc 2011; 286: 921–929.
Reference Link - Wannamethee SG, Shaper AG, Walker M. Physical activity and risk of cancer in middle-aged men. Br J Cancer 2001; 85: 1311–1316.
Reference Link - World Cancer Research Fund, American Institute for Cancer Research. Physical activity, In “Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective” (World Cancer Research Fund, American Institute for Cancer Research Ed.), Washington. 2007, pp. 198–209.
- Hagio M, Matsumoto M, Yajima T, Hara H, Ishizuka S. Voluntary wheel running exercise and dietary lactose concomitantly reduce proportion of secondary bile acids in rat feces. J Appl Physiol 2010; 109: 663–668.
Reference Link - Shephard RJ, Rhind S, Shek PN. The impact of exercise on the immune system: NK cells, interleukins 1 and 2, and related responses. Exerc Sport Sci Rev 1995; 23: 215–241.
Reference Link - McTiernan A, Ulrich C, Slate S, Potter J. Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control 1998; 9: 487–509.
Reference Link - Hoffman-Goetz L, Apter D, Demark-Wahnefried W, Goran MI, McTiernan A, Reichman ME. Possible mechanisms mediating an association between physical activity and breast cancer. Cancer1998; 83: 621–628.
Reference Link - Demarzo MM, Martins LV, Fernandes CR, Herrero FA, Perez SE, Turatti A et al. Exercise reduces inflammation and cell proliferation in rat colon carcinogenesis. Med Sci Sports Exerc 2008; 40: 618–621.
Reference Link - Song BK, Cho KO, Jo Y, Oh JW, Kim YS. Colon transit time according to physical activity level in adults. J Neurogastroenterol Motil 2012; 18: 64–69.
Reference Link - Aoi W, Naito Y, Takagi T, Kokura S, Mizushima K, Takanami Y et al. Regular exercise reduces colon tumorigenesis associated with suppression of iNOS. Biochem Biophys Res Commun 2012; 399: 14–19.
Reference Link - Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol 2001; 19: 816–827.
Reference Link - Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 2001; 107: 1049–1054.
Reference Link - Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 1995; 130: 503–506.
Reference Link - Jendraschak E, Sage EH. Regulation of angiogenesis by SPARC and angiostatin: implications for tumor cell biology. Semin Cancer Biol 1996; 7: 139–146.
Reference Link - Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem 2007; 282: 22062–22071.
Reference Link - Chlenski A, Guerrero LJ, Salwen HR, Yang Q, Tian Y, Morales La Madrid A, et al. Secreted protein acidic and rich in cysteine is a matrix scavenger chaperone. PLoS One 2011; 6: e23880.
Reference Link - Nie J, Sage EH. SPARC functions as an inhibitor of adipogenesis. J Cell Commun Signal 2009; 3: 247–254.
Reference Link - Nakamura K, Nakano S, Miyoshi T, Yamanouchi K, Matsuwaki T, Nishihara M. Age-related resistance of skeletal muscle-derived progenitor cells to SPARC may explain a shift from myogenesis to adipogenesis. Aging (Albany NY) 2012; 4: 40–48.
- Puolakkainen PA, Brekken RA, Muneer S, Sage EH. Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis. Mol Cancer Res 2004; 2: 215–224.
- Said N, Motamed K. Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. Am J Pathol 2005; 167: 1739–1752.
Reference Link - Yiu GK, Chan WY, Ng SW, Chan PS, Cheung KK., Berkowitz RS et al. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol 2001; 159: 609–622.
- Cheetham S, Tang MJ, Mesak F, Kennecke H, Owen D, Tai IT. SPARC promoter hypermethylation in colorectal cancers can be reversed by 5-Aza-2_deoxycytidine to increase SPARC expression and improve therapy response. Br J Cancer 2008; 98: 1810–1819.
Reference Link - Yang E, Kang HJ, Koh KH, Rhee H, Kim NK, Kim H. Frequent inactivation of SPARC by promoter hypermethylation in colon cancers. Int J Cancer 2007; 121: 567–575.
Reference Link - Tai IT, Tang MJ. SPARC in cancer biology: its role in cancer progression and potential for therapy. Drug Resist Updat 2008; 11: 231–246.
Reference Link - Tai IT, Dai M, Owen DA, Chen LB. Genome-wide expression analysis of therapy-resistant tumors reveals SPARC as a novel target for cancer therapy. J Clin Invest 2005; 115: 1492–1502.
Reference Link - Taghizadeh F, Tang MJ, Tai IT. Synergism between vitamin D and secreted protein acidic and rich in cysteine-induced apoptosis and growth inhibition results in increased susceptibility of therapy-resistant colorectal cancer cells to chemotherapy. Mol Cancer Ther 2007; 6: 309–317.
Reference Link - Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K. Altered expression of β-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis 2000; 21: 1319–1327.
Reference Link - Hojman P, Dethlefsen C, Brandt C, Hansen J, Pedersen L, Pedersen BK. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am J Physiol Endocrinol Metab 2011; 301: E504-E510.
Reference Link - Bortoluzzi S, Scannapieco P, Cestaro A, Danieli GA, Schiaffino S. Computational reconstruction of the human skeletal muscle secretome. Proteins 2006; 62: 776–792.
Reference Link - Chan XC, McDermott JC, Siu KW. Identification of secreted proteins during skeletal muscle development. J Proteome Res 2007; 6: 698–710.
Reference Link - Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics 2010; 9: 2482–2496.
Reference Link - Choi S, Liu X, Li P, Akimoto T, Lee SY, Zhang M, Yan Z. Transcriptional profiling in mouse skeletal muscle following a single bout of voluntary running: evidence of increased cell proliferation. J Appl Physiol 2005; 99: 2406–2415.
Reference Link - Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 2005; 19: 1498–1500.
- Guelfi KJ, Casey TM, Giles JJ, Fournier PA, Arthur PG. A proteomic analysis of the acute effects of high-intensity exercise on skeletal muscle proteins in fasted rats. Clin Exp Pharmacol Physiol 2006; 33: 952–957
Reference Link - Holloway KV, O’Gorman M, Woods P, Morton JP, Evans L, Cable NT et al. Proteomic investigation of changes in human vastus lateralis muscle in response to interval-exercise training. Proteomics 2009; 9: 5155–5174.
- Rooney K, Trayhurn P. Lactate and the GPR81 receptor in metabolic regulation: implications for adipose tissue function and fatty acid utilisation by muscle during exercise. Br J Nutr 2011; 106: 1310–1316.
Reference Link - Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 2007; 21: 2602–2612.
Reference Link - Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659.
Reference Link - McCarthy JJ. MicroRNA-206: the skeletal muscle-specific myomiR. Biochim Biophys Acta 2008; 1779: 682–691.
- McCarthy JJ. The MyomiR network in skeletal muscle plasticity. Exerc Sci Sport Rev 2011; 39: 150–154.
Reference Link - Small EM, O’Rourke JR, Moresi V, Sutherland LB, McAnally J, Gerard RD et al. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc Natl Acad Sci USA 2010; 107: 4218–4223.
- Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol 2004; 5: R13.
Reference Link