1. Natural 24-hour biological clock
Written around the edge were things like "Time to make tea",
"Time to feed the chickens", and "You're late!"
J. K. Rowling, Harry Potter and the Chamber of Secrets (1998)
Normal physiology usually has a rhythmical pattern to it, that is, different activities in the living cells normally occur every day and within the limits of fixed time intervals. For example, the morning cortisol release normally occurs between 6 and 8am; the output of diuresis is at its lowest at night and at its highest in the afternoon, the melatonin secretion usually starts at the beginning of the darkness phase (6–9pm), etc.). Circadian rhythm' (from the Latin phrase circa diem', that is, around a day') or circadian biological clock' are terms used to describe the periodically occurring changes in the expression profile, the biochemistry and the physiology of a cell, a tissue or an organism (e.g. the alternating rest-activity cycles; feeding and metabolism rhythms; daily changes in body temperature, pulse rate and blood pressure; nervous system activity; hormone release; elimination of waste, etc.). The levels of the majority of the mRNAs, proteins and metabolites in eukaryotic cells rhythmically rise and fall in a cyclical fashion. The length of the complete cycle is usually close to 24 hours.
According to the definition given by Beatrice Sweeney, one of the pioneers in the field of research of circadian rhythms, "A circadian rhythm is an oscillation in a biochemical, physiological, or behavioural function which under conditions in nature has a period of exactly 24 hours, in phase with the environmental light and darkness, but which continues to oscillate with a period of approximately but usually not exactly 24 hours" [
The phrase biological clock' (often used interchangeably with circadian clock' or circadian rhythm') could be heard so often nowadays, and in contexts so unrelated to science that it is hard to remember that it had always had a strictly defined meaning. In simpler words, it is the biological clock that reminds us that several hours have passed since we have last eaten (feeding time); that 7–9 hours have passed since we have fallen asleep at night (time to wake up); that we have gone without sleep for 16 or more hours (time to go to bed), etc. For some reason, the term biological clock' is popularly (and incorrectly) used as a connotation of the mechanism of ageing (and most commonly – as a reference to the upper limit of reproductive age of women (e.g. "her biological clock is ticking fast, she is desperate")). In order to avoid any such confusion, we will briefly outline the difference between the basic types of biological clocks.
In principle, a biological clock may belong to one of two basic types: 1) oscillators (as is the circadian clock and similar clocking mechanisms that normally work in cycles shorter than 24-h – 12-h, 8-h, etc. (intradian clocks)); and 2) unidirectional (hourglass) clocks (telomere attrition rate, the Hayflick's limit, etc.). Oscillating clocks usually regulate the normal day-to-day functioning of the cell, the tissue and the organism in young as well as in old age. Indeed, as we will see below, as age advances, the regularity of circadian rhythms becomes impaired and more prone to resetting or disruption by minor stimuli, with multiple deleterious consequences. Unidirectional clocks measure the time until individual cells (and, in a more complex manner – the tissues and the organism) are decommissioned by the mechanism of replicative senescence/death of old age.
Many of the normal physiological processes follow a rhythmical circadian pattern, and many aspects of daily life may revolve around circadian changes. For example, the levels of cortisol in mammals and man peak every day almost immediately after waking up from nighttime sleep, enhancing the stress response in the early wakefulness phase. This may be manifested by heightened arousal, including hypersensitivity to various stimuli and/or hypervigilance (the well-known early morning irritability'). The cortisol awakening response may be enhanced by acute and chronic sleep deprivation (having not slept the previous night or and/or chronic loss of sleep); need to awake very early in the morning (e.g. in shift workers taking very early-morning shifts); consumption of alcohol, caffeine, and other substances; and/or stress, producing anxiety and jitteriness, and, sometimes, volatile temper [
In higher eukaryotes, including mammals and man, the central circadian time' is set by the master circadian clock. It is located in the suprachiasmatic nuclei (SCN) in the hypothalamus [
Every eukaryotic cell possesses the requisite molecular machinery of the core circadian clock and may follow the physiologic rhythm for a long time, even in the absence of major cues. The maintenance of the circadian rhythm, however, is better sustained on tissue, organ and organism levels than in single cells, as the correspondence between the central and the peripheral clocks is necessary to ensure the subtle adjustments of the circadian rhythm. Typically, the clock of the organism is rapidly reset in response to specific cues, but cultured cells may rapidly lose the circadian rhythm, sometimes even in the presence of potent clock-adjusting cues [
The external cues capable of adjusting the circadian clock (also called Zeitgebers', or time givers') may be endogenous or exogenous. A prime example of exogenous clock-adjusting factor is light. The master clock in the mammalian brain is exquisitely sensitive to light. Even a single short light pulse may suffice for resetting the master clock, provided that the wavelength of the light is shorter than the wavelength of infrared light. The light is perceived by the photoreceptors in the retina; then the signal is transmitted to SCN and subsequently to the pineal gland. The latter is the topological site where melatonin is produced. Melatonin (N-acetyl-5-methoxytryptamine – a tryptophan derivative) is the major hormone regulating the sleep-wakefulness cycle as well as other physiological processes, such as sexual development, sexual behaviour, etc. Melatonin is usually produced in small quantities during the daylight phase of the 24-h cycle in diurnal animals and man and in larger quantities during the darkness phase as light suppresses its synthesis [
Exogenous melatonin may cause sleep phase advancement [
Some hormones may function as endogenous cues for adjustment of the circadian clock. For example, administration of the synthetic cortisol analogue dexamethasone may reset the peripheral circadian clocks in mice [
The exact time at which a phase (e.g. rest phase/activity phase) occurs in the 24-h cycle may vary (sometimes – significantly) between individuals of the same species. Among humans, there have always been people that feel the need to go to bed early in the evening and wake up refreshed and alert early in the morning (the morning lark' chronotype); whereas others feel their best in the late afternoon and evening, do not go to bed until late after midnight and sleep soundly until noon the next day (the night owl' chronotype). Extreme variants of the morning lark' and night owl' chronotypes may be synonymous to advanced and delayed sleep phase disorder. Misalignment between the time in the subjective day when one really must get up (e.g. to go to school or to work) and the time one feels rested; and between the time when one needs to go to bed because they are too tired to stay awake and the time when they ought to go to bed in order to ensure that they have rested for enough hours may cause problems. The latter, however, usually become significant only in the cases when the time points between "desired time" and "actual time" for a phase switch are desynchronised by three or more hours (e.g. one must get up at 7am to get to work on time, therefore, they ought to be in bed by 11pm; but one can't normally go to sleep before 2am and does not feel their best before noon). Nevertheless, the fact that the timing of the rest/activity cycle may be quite different in different people has been brought to public awareness to the point that more flexibility in planning study and work cycles are currently being advocated to ensure than individuals with either of the basic chronotypes have equal chances for performing well in school, at the workplace, and in specific cases, such as examinations and business meetings [
The chronotype is heritable, and in specific cases (see below) the molecular variant implicated in chronotype establishment may be identified. It is, however, moderately changeable and may be learned or 'transitioned to', given enough time. Daily routine may play a major role in the adjustment and even the complete reversal of the chronotype. The fact remains, however, that the chronotype of parents is often similar or identical to the chronotype of the offspring. The latter formed the basis of a hypothesis about the possibility of assortative mating among people with morning' and evening' chronotypes', based on the assumption that persons with the one chronotype could only seldom meet persons with the other chronotype, as their daily routines would dovetail' rather than match [
Frequent changes of the daily routine causing disruption of the circadian rhythm (circadian misalignment) – e.g. travelling across several time zones (jet lag), taking rotating shifts, etc.; acute or chronic stress; or failure to entrain the biological clock because of misguiding environmental cues (e.g. working during the day in badly lit offices and sleeping in the light-polluted environment of large cities) may affect the human organism in many ways. Circadian rhythm disruption may bring about various conditions associated with day/night cycle reversal (chronic insomnia at night and/or daytime sleepiness); disordered food intake and energy expenditure (e.g. undesired weight gain); troubled digestion; hormonal dysregulation and others (for details, see below). Notably, these adverse effects may be independent of sleep deprivation, that is, they may develop even in individuals that get their 7–9 hours of sleep per 24h and their minimum dose of light when they are awake, but these occur 'at the wrong time' of the day. There is also the well-documented condition of 'midwinter insomnia' affecting people living in territories close to the North polar circle, where the sun does not rise above the horizon for weeks and months in the winter [
In some cases, disruption of the circadian rhythms may be dangerous. It infamously known that working at night, especially performing monotonous tasks in a badly lit environment (e.g. driving) may result in occurrence of very short (lasting only a second) episodes of falling asleep, which may have very grave consequences. This may occur regardless of whether the driver had slept enough before they sat behind the wheel or not (albeit is it more likely to occur if they have not).
Chronic disruption of the rhythm set by the internal clock may put the individual at increased risk for some common diseases with multifactorial genesis. Circadian misalignment may be associated with increased risk for development of insulin resistance (metabolic syndrome, diabetes type 2) [
2. Looping the loop – core machinery of the circadian clock
On a single-cell level, the functioning of the circadian clock is based on periodical oscillations in the levels of several proteins, forming a negatively controlled feedback loop [
The Cry proteins (from cryptochrome') – Cry1 and Cry2 and the Per proteins (from period') – Per1, Per2 and Per3 are negative regulators of the core circadian clock in mammals. Per and Cry proteins also function together, forming heterodimers. The Per/Cry complex switches the expression of Clock and Bmal1 off, downregulating its own transcription as well (Fig. 1) [
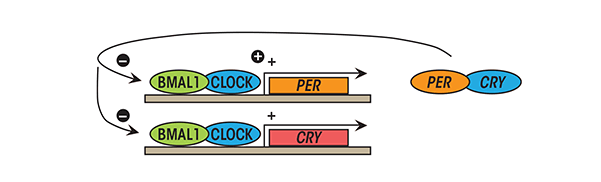
Bmal1, Cry and Per proteins have half-lives of only about 3 hours, as they are continually tagged for degradation by E3 ubiquitin ligases [
Npas2 may substitute for Clock in the suprachiasmatic nuclei [
The promoters of the genes regulated by the circadian clock are usually GC-rich [
Clock and Bmal1 proteins are members of the bHLH-PAS family of proteins. They contain a basic helix-loop-helix (bHLH) domain at the N- terminus [reviewed in detail in
The levels of Clock normally oscillate within the 24-h cycle in eukaryotic cells, but the mechanisms for the rhythmical increase and decrease of the levels of Clock mRNA and protein may be different in different species. The levels of expression of Clock remain relatively steady throughout the 24-hour period in the mouse, but the levels of its mRNA and protein may vary significantly between the nuclear and the cytosolic compartment at different times of the day [
The Per/Cry dimer usually suppresses the transcription of day-phase' proteins by binding to their E-box sequences. The peaks of expression of Per and Cry usually occur in anti-phase to the expression peaks of Clock and Bmal1 (Npas2), although the timing may be different for different proteins. In rats Per1 mRNA peaks in early morning, Per3 mRNA – late in the afternoon and Per2 mRNA – in the transition period between the day and the night (at dusk) [
Cry1 and Cry2 may have light-dependent as well as light-independent regulatory functions. The Cry1 gene contains a day-time' element (D-box) as well as night-time' elements (Rev-ErbA/ROR response elements, RRE) [
The proteins of the eukaryotic circadian oscillator are highly conserved, even between distantly related species. Eukaryotic cryptochromes are related structurally and phylogenetically to Class I prokaryotic photolyases [
Besides the core clock machinery, there are also other proteins functioning in the regulation of the circadian rhythm in higher eukaryotes – e.g. Tim1 (Timeless); deleted in oesophageal cancer (Dec) – Dec1 and Dec2; nuclear receptor subfamily 1 group D (Rev-Erb) proteins; retinoic acid-related orphan receptors (RORs), and others [
There are usually several hours of delay between the peak in the mRNA level of core clock genes and the peak of the expression of the corresponding protein. The core clock feedback loop is controlled predominantly by the slower transcription-mediated mechanism, as the associated mRNA and protein peaks occur within several hours of each other and the timings of peak and trough levels are normally predictable. However, the core proteins of the clock machinery are subject to modification at post-translational level [
Circadian cycles have probably arisen early in evolution, as all eukaryotic cells and even some prokaryotes (e.g. Cyanobacteria) exhibit a 24-h rhythmicity in many of their functions. Cyanobacteria are among the oldest organisms currently living on Earth. The Cyanobacteria system for maintenance of the circadian cycle consists of only four proteins – the three proteins of the kai cluster (kaiA, kaiB, and kaiC) and the sensory histidine kinase sasA [
3. Circadian rhythm of the cell cycle
Publius Ovidius Naso, Amores, c. XVI century BC, Book I, XIII, Line 40.
The synthetic phase of the cell cycle in eukaryotic cells occurs predominantly at night (although this may vary in different tissues). Timing of replication to the darkness phase of the day/night cycle is believed to be an adaptive mechanism originating from the early times of eukaryotic life on Earth (the 'escape from light' hypothesis) [
The circadian rhythmicity of entry and exit from the different phases of the cell cycle is especially noticeable in tissues with rapid turnover. For example, dividing cells from colonic mucosa normally enter the G1 phase of the cell cycle in a relatively coordinated fashion in late afternoon/early evening and the percentage of cells in S phase peaks around midnight [
There are tissues in which the different phases of the cell cycle occur at times inconsistent with the general rule that replication occurs at night. For example, in tissues that are well protected from genotoxic effects (e.g. haematopoietic progenitors in the bone marrow - protected by layers of connective tissue, fat and bone and very rarely coming in contact with genotoxic agents) the replication is normally timed during the day. This may be observed in species with differently timed activity/rest cycles. For example, the synthetic phase of the cell cycle in the bone marrow of mice occurs predominantly in the morning, with the mitotic peak occurring in the interval 6am–12am [
The skin, as the outer border of the multicellular body, is subjected in full to the environmental changes typical of the day/night cycle (ambient temperature, levels of UV irradiation, etc.). The proliferation of the progenitor cells in adult epidermis (healthy as well as damaged) occurs in an oscillating fashion, with the completion of the cell cycle significantly accelerated (3–5 times) in the early phases of skin regeneration. In the mouse epidermis, the S-phase occurs predominantly late into the night [
Melatonin is a potent free radical scavenger [
The expression of the genes of the core circadian clock may be developmentally regulated. The cell cycle of undifferentiated cells (e.g. cells of the early embryo, pluripotent stem cells) is probably not controlled by the circadian clock, at least until the onset of differentiation. In mouse embryonic stem cells, the rhythmicity of metabolism is established before the rhythmicity in the expression of the core clock genes [
The timing of the phases of the cell cycle of adult stem cells and progenitor cells also follows a circadian pattern. The rhythmic waves of transcription of the core clock genes in adult epidermal stem cells and in dividing keratinocytes coincide with peaks of expression of specific subsets of genes involved in the proliferation and differentiation of keratinocytes (e.g. KLF-9) and in their capacity to respond to environmental cues (e.g. TGF-beta) [
Undifferentiated cells from rodents and stem cells from primates and man may exhibit very different properties, specifically in the pattern or repair of DNA damage and the cell cycle [
Cancer stem cells may retain the circadian rhythm of cell division, but the properties of the circadian rhythm in them may be somewhat different from the properties in normal cells. Murine cancer stem cells do not exhibit the typical rhythmical pattern of localisation of Per2 to the cell nucleus [
Differentiation of precursor cells may also follow a circadian pattern. In mouse oligodendrocyte precursors the cell division occurred predominantly during the rest (daylight) phase, whereas the cell differentiation occurred during the wakefulness (nighttime) phase [
The expression of core clock genes and clock-controlled genes in cancer cells may significantly deviate from the typical pattern of expression in normal cells. The expression of some of the genes of the core clock machinery may follow a circadian pattern different from the rhythm of nontransformed cells, others may be homogeneously expressed; or their expression may be inhibited or altogether lost (for details see below). Early research in biopsy samples from different types of tumours showed that some types of tumours (e.g. squamous carcinomas in mice and colorectal carcinomas in man) exhibited no variation in the growth rate within the 24-h cycle [
4. Presence of damage in DNA is a potent cue for adjustment of the circadian clock
Persistence of unrepaired damage in DNA in a normal (nontransformed) cell, preparing for division, results in cell cycle arrest, damage assessment, and attempts for repair of damage and/or induction of apoptosis. Acknowledgement of the presence of unrepaired damage in DNA may trigger adjustment of the circadian clock to a time in the 24-h cycle when the routine checks on DNA integrity, damage repair and/or induction of apoptosis are normally carried out (phase shift). Only after the damage is repaired the cell may attempt to pass the cell cycle checkpoints again.
The first experiments showing the potential of DNA damage as a trigger for phase shifting were carried out on dinoflagellates (single-cell phototrophic eukaryotes capable of producing light) – specifically, Gonyaulax polyedra. Irradiation of G. polyedra cultures with white light or monochromatic light from the visible spectrum during the 'low luminescent capacity' phase (daytime) results in enhanced luminescence in the 'high luminescent capacity phase' (at night), with the rhythm continuing steadily for several days when the cultures were kept constantly in the dark, although the amplitude of the rhythm declined after 3–4 days of constant darkness [
Both major damage-associated signalling pathways (p53-associated and ATM-associated) are capable of induction of circadian phase shifts. DNA damage normally dealt with by activation of ATM/ATR-dependent pathways (strand breaks) usually produces advancement of the next phase of the circadian cycle, with the magnitude of the advance dependent on the subjective time of day when the damage occurred. The mechanism is conserved between different species.
In Neurospora crassa, treatment with DNA damaging agents causes advancement of the next circadian phase [
Per1 and Per2 proteins of the mammalian core clock play important roles in the regulation of ATM-Chk2/ATR-Chk1-associated damage response pathways [
Damage that activates the p53-regulated pathways also produces phase advancements. Some of the core clock genes are directly regulated by p53. For example, the promoter of Per2 contains a conserved p53-response element that partially overlaps the E-box where the Bmal1/Clock dimer normally binds. In the presence of damage, p53 bound to the response element in Per2 gene blocks Bmal1/Clock dimer binding to the Per2 promoter, inhibiting the expression of Per2. This results in advancement of the next circadian phase (in which the levels of Per2 are naturally low) [
Many of the p53-associated effects on the circadian cycle are exerted indirectly. The transcription from the Bmal1 promoter was found to be enhanced in the presence of DNA damage, advancing the onset of the next circadian phase [
p53-deficient mice are tumour-prone and short-lived. They cycle normally between the rest/activity phases when housed in complete darkness but the period is shorter than 24h and may be unstable. Light pulses normally rapidly adjusts the core clock in mice kept in complete darkness, causing phase advances or phase delays, depending on the time in the circadian cycle when the light pulse was administered (e.g. a light pulse administered during the subjective night may cause advance of the next (daylight) phase whereas a pulse administered at the end of the subjective day may delay the onset of the nighttime phase). p53-deficient mice exhibited longer phase delays in response to a light pulse than wild type mice and failed to respond to light with phase advances [
5. Circadian oscillations in the levels of gene products directly involved in the regulation of cell division and/or the transition through major cell cycle checkpoints
The devil will come, and Faustus must be damn'd.
Christopher Marlowe, Doctor Faustus (1604), Scene XIV, Line 36.
The levels of expression of many proteins associated with checkpoint transition, progression in the cell cycle and/or induction of apoptosis may vary within the 24-h cycle. Among the clock-regulated proteins are the c-Myc family of DNA-binding proteins; checkpoint kinases, cyclins and other positive regulators of the cell cycle; negative regulators of the progression in the cell cycle – e.g. CDK inhibitors (p21 (Waf1), p16 (INK)) and Gadd45; proteins functioning in DNA damage-associated response pathways and the maintenance of genomic integrity (ATM, p53, HMG-family of proteins); pro-apoptotic proteins (e.g. the pro-apoptotic members of the BCL-2 family – Bax, Puma); and others. The expression of these proteins may be directly regulated by the core circadian clock (usually, via E-boxes – e.g. c-Myc, Xpa, and others), or they may be downstream targets for transactivation by the core clock genes, their levels following (usually, with only a small delay) the oscillation pattern of the levels of the core clock proteins.
The expression of p53 does not follow a circadian rhythm, as p53 is a major player in many and varied processes in living cells and must respond differentially to events causing its stabilisation and accumulation [
The expression of c-Myc protein is controlled directly by the proteins of the core clock. c-Myc has affinity to E-box sequences in its target proteins [
The expression of cyclins oscillates within the circadian cycle. The levels of expression of cyclins D1 and E in colonic mucosa typically reach their peak levels in late afternoon/early evening (6pm) before G1/S checkpoint transition; and their lowest levels around midnight, when the M phase is about to begin [
The expression of some of the essential proteins of the complexes recognising DNA damage and implementing DNA repair may vary rhythmically within the 24-h cycle [
Proteins functioning in DNA repair other than NER may be controlled by the circadian clock. The levels of N-methylpurine DNA glycosylase (a BER glycosylase, excising 3-methyladenine and 7-methylguanine from DNA) and O6-methylguanine-DNA-methyltransferase (an enzyme catalysing the direct repair of methylated bases by transferring the methyl groups to its own molecule) also oscillate within the 24-h cycle. The peak and the trough levels of these enzymes, however, rarely differ by 1.5–2 -fold [
Some of the core clock proteins may directly influence the chromatin architecture and dynamics. Clock protein possesses intrinsic histone acetyltransferase activity, acetylating the conserved Lys14 residue of histone H3 as well as other (nonhistone) targets, including its own pairing partner Bmal1 (again, on specific lysine residue – Lys537) [
The levels of some of the proteins functioning in the maintenance and the remodelling of the chromatin structure, such as HMG, may rise and fall rhythmically in the 24-h cycle. Hmgb1 levels oscillate in the retinal photoreceptor cells in rats, with protein levels peaking at midday and reaching their minimum late at night [
The levels of Gadd45α and Gadd45β (proteins involved in damage-associated inhibition of the entry in S-phase of the cell cycle and induction of apoptosis) also oscillate rhythmically during the day [
The levels of pro-apoptotic proteins and proteins of DNA damage-associated response normally oscillate within the 24-h cycle, but the amplitude of the oscillation may be significantly altered in cells that have sustained damage. For example, gamma-irradiation of mouse haematopoietic progenitor cells from bone marrow causes significant increases in the oscillation amplitudes of mRNA and protein of the CDK inhibitor p21 and the major negative regulator of p53, Mdm2. The time points in the circadian cycle when the damage had occurred may also significantly matter. If we may use the above example, gamma-irradiation of haematopoietic progenitors from mouse bone marrow occurring early in the night resulted in higher p21 and Mdm2 mRNA levels than the maximum levels of p21 and Mdm2 achieved when the irradiation occurred during the day [
Differences in the amplitude of the circadian oscillations of the levels of expression of genes associated with response to DNA damage may also exist between cells of the same basic type at different phases of differentiation. Using the above example again, when the levels of the proteins p21, Mdm2, Bax and Puma were quantitated in cells isolated from peripheral blood instead of bone marrow cells, the magnitude of the increase in the levels of p21, Mdm2, Bax and Puma was almost two times higher after irradiation occurring during the day than the levels achieved after nighttime irradiation [
6. Genotype-phenotype correlations for mutations and polymorphisms in genes coding for products of the core circadian machinery
The majority of the studies on the functioning of circadian clocks in vivo were carried out in animal models (Drosophila, rats and mice). Human studies on circadian rhythmicity are exclusively observational. Experiments involving specific setups that may cause disruption of circadian rhythms in human beings are considered unethical, as they entail prolonged periods of complete darkness and/or constant illumination, and because studies have so far shown that the health consequences may be quite serious. The reliability of currently available data about the impact of the disruption of circadian rhythm in humans may, therefore, be questionable. For example, in many earlier studies on the impact of light at night and insufficient lighting during the day the participants were allowed to switch artificial light on and off whenever they wanted to, despite its potential role in the resetting of the circadian clock [
6.1. Animal models
Mouse models of deficiencies of virtually all genes of the core circadian loop (or variant alleles of these genes) have been established. Not all of these models exhibit altered circadian pattern (cycle shorter or longer than 24 h) when housed in the absence of environmental cues (e.g. constant darkness) or loss of circadian rhythmicity [reviewed in
The phenotype of Bmal1-deficient homozygotes is the most severe of all mouse phenotypes conferred by mutations in circadian clock genes. While loss of both copies of Clock or both copies of any of the Per and Cry genes does not disrupt the circadian rhythms in mice housed constantly in the dark (although it may cause changes in cycle duration), loss of the Bmal1 gene results in almost immediate and complete loss of circadian rhythmicity in constant darkness [
Npas2 deficiency in mice results in slightly shortened circadian cycle without obvious features of accelerated ageing and/or cancer proneness [
Mice carrying mutations in the Clock gene experience lengthening of the circadian cycle and sleep phase delay [
Both genes coding for Per1 and Per2, and Cry1 and Cry2 must be disrupted at the same time to produce a phenotype of deregulation of circadian rhythms in the mouse, as the functions of the protein products in each pair of homologues may partially overlap [
Homozygous Cry1 deficient mutants usually experience cycle shortening when housed in complete darkness, whereas Cry2 homozygous mutants exhibit lengthening of the circadian cycle [reviewed in
Cry1 homozygous knockout mice, Cry1/Cry2 double knockout mice and Per2 homozygous mutant mice are cancer-prone (spontaneously or following genotoxic challenge) [
There is an intriguing interference between the deficiency of Cry1/Cry2 in mice and the carriership of inactivating mutations in the Tp53 gene. Both conditions, when inherited independently, result in cancer proneness and decreased lifespan [
6.2. Human phenotypes associated with carriership of polymorphic variants of core clock genes
Some rare disorders characterised by abnormal timing of sleep phase and/or duration may be associated with carriership of variant alleles of genes coding for proteins of the circadian clock. The Ser662Gly mutation in the human PER2 gene (rs121908635, associated with advanced sleep phase syndrome 1) modifies a phosphorylation site in the PER2 protein [
The natural short sleeper' human phenotype is associated with heterozygous carriership of the mutation Pro385Arg in the DEC2 gene [
The C allele of the 3111C/T noncoding polymorphism (rs1801260) in the 3'-UTR of the human CLOCK gene has been associated with eveningness' (that is, feeling best and fittest in the hours of afternoon to evening/night, usually accompanied by sleep phase delay), as opposed to morningness', which is characterised by preference for the hours of early morning to early afternoon and sleep phase advancement [
An association between the variant (A) allele of the Rev-ErbA1 polymorphism rs2314339 (G>A) and the risk for abdominal obesity was identified in two different populations (specifically, of Spanish Mediterranean and of North American origin). Specifically, the risk for abdominal obesity was estimated to be about 1.5 times lower for individuals with A-allele containing genotypes (AA, AG) than in G-allele homozygous carriers. The Rev-ErbA1 rs2314339 genotype did not correlate with energy intake (eating patterns, dietary preference) [
Inherited polymorphisms in core clock genes may constitute a genetic factor associated with increased risk for development of various tumours. Carriership of the 3111C/T polymorphism in the human CLOCK1 gene has been linked to increased susceptibility for colon cancer [
7. Association between the disruption of circadian rhythm and the risk of occurrence of common diseases and conditions (diseases of middle and advanced age)
Since many physiological processes occur rhythmically within the 24-h cycle, it could be expected that the disruption of the circadian rhythm may increase the risk for development of multiple diseases and conditions. Indeed, data from mouse models (see above) suggests that the dysfunction of the basic players in the constitution of the circadian rhythm may increase the risk for metabolic syndrome, diabetes, cancer and cardiovascular disease. The International Agency for Research on Cancer (IARC) published in 2007 a statement to the effect that "... Shiftwork that involves circadian disruption is probably carcinogenic to humans..." [IARC shiftwork statement]. Industrialisation is unavoidably associated with disruption of the day/night cycle as it increases the proportion of people involved in indoor jobs during the day (which means insufficient amounts of daylight) and staying up late at night in an environment saturated with artificial lighting. Typical evening activities in modern societies are usually associated with consumption of the major meal of the day late in the evening (or even the night) and increased frequency of snacking between meals (usually, on foods rich in carbohydrate and fat). To this adds the effect of night shift work and the opportunities for travelling long distances by air flight (associated with jet lag syndrome). It could be expected that the effect of disrupted circadian rhythms on human health would greatly increase in the near future.
Numerous studies conducted in humans showed that disruption of the physiological circadian rhythm may significantly increase the risk of various tumours - specifically, cancer of the mammary gland, the prostate gland, the colon and rectum; and the lung [
The potential for adjustment of the core clock by external cues (light) may also play a role in the constitution of cancer risk. The overall incidence of cancer has been reported to be lower among the totally blind compared to the general population [
The cancer risk conferred by disruption of circadian rhythms may be modifiable by other factors, including other risk factors. For example, there have been reports about increased risk for lung cancer among selected groups with chronically disrupted circadian cycle (night shift workers) [
Besides the impact on the risk for development of cancer, disruption of circadian rhythms may be partially responsible for differential outcomes when cancer has already developed. Studies conducted in mice demonstrated that light at night may stimulate the growth of carcinoma of the mammary gland [
In some tumours (the already mentioned colorectal, breast and prostate cancers, but also in thyroid carcinoma) have been observed somatic mutations of the core genes of the circadian clock and/or the auxiliary clock genes [
The expression of DEC1 is severely downregulated or the gene copies are deleted in over 50% of the cancers of the oesophagus [
Studies conducted in patients with chronic lymphocytic leukemia (CLL) showed that in tumour cells the expression of BMAL1, PER1 and PER2 was downregulated, whereas the expression of c-MYC and cyclin D1 was upregulated [
The levels of expression of core clock proteins may serve as predictors of individual outcomes in cancer. High BMAL1 levels in the primary tumour in patients with colorectal cancer were associated with longer overall survival (>1.5 times) than the overall survival rates in patients with tumours with low BMAL1 levels [
High levels of expression of CRY1 in patients with colorectal cancer were associated with lower overall survival rates [
Disruption of the oscillating rhythm of expression of some of the core clock proteins may be associated with more rapid cancer progression. For example, homogeneous (non-oscillating) expression of BMAL1 or PER2 in breast cancers may be associated with increased rates of lymph node metastasis and more aggressive course of the disease [
It has been proposed that the growth rate of tumours was different at different times of the 24-h cycle [
The circadian clock has been found to play a role in the pathogenesis of multifactorial diseases and conditions other than cancer as well. As was already mentioned above, disruption of the circadian rhythm in animals and man has been linked with deregulation of feeding patterns/energy expenditure and with disordered glucose metabolism. Weight gain and/or failure to lose weight are common consequences of the disruption of the day/night rhythm [
Environmental factors other than light, temperature and food intake may directly or indirectly affect the circadian rhythm. Such factor is, for example, tobacco smoke. BMAL1 was rapidly acetylated and subsequently degraded in the lungs of mice exposed to cigarette smoke and in human patients with chronic obstructive pulmonary disease (COPD), compared with non-smoking mice and COPD-free human controls [
Normally, the pulse rate and blood pressure significantly decrease (dip) at night, when one's asleep. Individuals that do not experience the nighttime drop in blood pressure (non-dippers) are believed to be at elevated risk for vascular incidents [
The short-term and long-term consequences of brain injury often include disruption of the day/night rhythm [
The levels of cortisol and melatonin may significantly change before and after surgery, not only because of the activation of the stress response, but also because of specific effects of general anaesthesia. The sleep disorders commonly seen in postoperative patients are usually related to circadian rhythm disruption produced by deregulation of cortisol and body temperature homeostasis, as well as by direct deregulation of melatonin synthesis [
Disruption of the normal circadian rhythm is a very common finding in mood disorders and the expression of core clock genes may be drastically altered in patients with depression [
The circadian clock may be responsible for the diurnal oscillations in the inflammatory responses to acute infection. It is known that some of the markers for infection oscillate during the 24-h cycle (e.g. different types of fever may regularly peak at the same time every day). This is usually attributed to features of the germinative cycle of the infectious agent. Nevertheless, it was shown that mice infected with Salmonella typhimurium showed higher rates of bowel and spleen colonisation when infected early in the rest period (in the morning) compared with mice infected in the evening [
The circadian pattern of expression of core clock genes shows signs of gross deregulation in aged cells [
The melatonin levels are decreased, the circadian rhythm in melatonin secretion is lost and rest-activity rhythm is grossly disturbed in patients with Alzheimer's disease [
Excessive daytime sleepiness commonly seen in patients with Parkinson's disease is believed to be associated with disturbances in resetting of the master clock by light cues and functional disengagement of the secretion of melatonin by the pineal gland from the rhythmic signals set by the master clock, eventually resulting in decreased amplitude between the daytime and night time levels of melatonin [
8. Putting chronobiological concepts at work - chronological nutrition and cancer chronotherapy
8.1. Snack around the clock, or how chronological nutrition reinvented normal eating
With the emergence of the concept of healthy' or successful' ageing, there have been many suggestions about how to increase the chances of being among the lucky few' that preserve their mental and physical capacities until very old age. Some of these suggestions make sense, other are, at best, questionable [
8.2. Timing anticancer treatments to the circadian rhythm in order to achieve a good therapeutic response with minimum adverse effects - cancer chronotherapy
The risks for occurrence and the severity of the adverse effects in anticancer therapy may be different when the same genotoxic agents are administered at different times within the 24-h cycle. This is, in a nutshell, the basis of chronotherapy (abbreviated from chronomodulated therapy), one of the modern branches of anticancer therapy. As was already mentioned above, the timing of the different phases in the cell cycle in cancer and non-cancer cells of the same tissue may significantly vary (or even in cases when it varies only slightly, it might be just enough to make all the difference). Cancer chronotherapy attempts at synchronisation of the delivery of the genotoxic agent to the peak of DNA synthetic activity in cancer cells, so as to achieve maximum inhibitory effect on tumour growth and (hopefully) much less severe suppression of the proliferation of normal cells. Even in cases when the peaks of DNA synthetic activity tend to overlap between cancer and non-cancer cells of the same tissue, the (presumably) much higher proliferative activity of cancer cells would ensure that they are affected by the genotoxic agent more than normal cells. Chronotherapy is believed to be associated with lower risk for therapy-associated adverse reactions, or, if the adverse effects occur, they are expected to be milder [
As with chronological nutrition, the concept of chronotherapy is not a modern invention. The first documented cases of cancer chronotherapy in human patients intended to achieve maximum response with minimum adverse effects date back to the 70-ties of the XX century [
As anticancer therapy developed steadily in the last decades of the XX century and the first decade of the XXI century, the hypothesis of scheduling genotoxic treatments to the times of the day when cancer cells were most susceptible to damage was supported with more experimental results. Nevertheless, definitive data illustrating the benefits from timed anticancer treatments began to be published only in the late 90-ties of the XX century. The largest body of information obtained so far is for metastatic colorectal cancer treated with oxaliplatin-5-fluorouracil-leucovorin combined regimens (FOLFOX) or irinotecan-5-fluorouracil- leucovorin (FOLFIRI). The first results of chronomodulated cancer therapy were highly encouraging. The objective response to therapy and the median survival were found to be significantly higher in patients treated with chronomodulated FOLFOX combined regimen for metastatic colorectal cancer than with conventional (flat-infusion) FOLFOX [
Timing of delivery of irinotecan as a single agent was also found to be important for the individual tolerability of the drug (at present – in animal models). The tolerability of irinotecan in mice with osteosarcoma was found to be at its best at Zeitgeber time (ZT) 15, and at its worst at ZT3 [
Nevertheless, similarly to the relationship between the individual capacity for DNA repair, the response to anticancer therapies and the risk for toxicity effects; the association between the chronomodulated vs. conventional therapy and the outcomes in terms of response and adverse effects turned out to be not that straightforward as they initially looked. Some of the studies in patients with metastatic colorectal cancer receiving the same combined regimen (FOLFOX) did not elicit significant differences between the survival rates of patients receiving the same regimen in conventional and chronomodulated settings [
The occurrence and the severity of adverse effects of genotoxic therapy may have radically different prognostic value as markers for therapeutic response in chronomodulated and conventional therapy. For example, it is known that the occurrence of adverse effects, such as neutropenia in patients receiving anticancer treatments is not uncommon and may be dangerous, even life-threatening. However, in patients on conventional chemotherapy neutropenia may be a predictor of good treatment response – presumably because high toxicity may indicate that the proliferation of tumour cells is also severely affected by the genotoxic treatment. It was reported that the development of severe adverse effects, such as neutropenia, fatigue and weight loss in the course of FOLFOX for metastatic colorectal cancer was associated with better survival in patients on conventional regimens, but poorer survival in patients on chronotherapy [
There are also other unexpected effects of chronotherapy with regards to dissimilar responses in different groups of patients. Women often have better survival rates than men after various diseases and may have better responses to different therapies. This may be related to the fact that women usually stick to the prescribed treatment schedules and tend to drop off any kind of treatment less often than men. It is, therefore, notable that men were reported to fare better than women in the majority of studies of cancer chronotherapy, with respect to therapy-related toxic effects and the outcomes after therapy [
Targeted stimulation or suppression of the expression of proteins of the core circadian clock may find potential use in the therapy of common human diseases in the near future. It has been repeatedly reported that sensitivity to anticancer therapies may be modulated by the expression levels of core clock genes. In human cancer cell lines transfected with PER1 the proportion of cells in S-phase decreased rapidly, whereas the proportion of cells in G2/M phase increased [
Acknowledgеments
This research was supported by Grant No. DFNI-B01/2 at the National Science Fund, Ministry of Education and Science of Republic of Bulgaria.
References
- Sweeney BM. Freeze-fracture studies of the thecal membranes of Gonyaulax polyedra: circadian changes in the particles of one membrane face. J Cell Biol. 1976; 68(3):451-61.
- Abe H, Rusak B. Anticipatory activity and entrainment of circadian rhythms in Syrian hamsters exposed to restricted palatable diets. Am J Physiol. 1992; 263(1 Pt 2):R116-24.
- Cambras T, Vilaplana J, Díez-Noguera A. Effects of long-term restricted feeding on motor activity rhythm in the rat. Am J Physiol. 1993; 265(2 Pt 2):R467-73.
- Wüst S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000; 25(7):707-20.
- Federenko I, Wüst S, Hellhammer DH, Dechoux R, Kumsta R, Kirschbaum C. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004; 29(2):174-84.
- Mirmiran M, Koster-Van Hoffen GC, Bos NP. Circadian rhythm generation in the cultured suprachiasmatic nucleus. Brain Res Bull. 1995; 38(3):275-83.
- Bernard S, Gonze D, Cajavec B, Herzel H, Kramer A. Synchronization-induced rhythmicity of circadian oscillators in the suprachiasmatic nucleus. PLoS Comput Biol. 2007; 3(4):e68.
- Porterfield VM, Mintz EM. Temporal patterns of light-induced immediate-early gene expression in the suprachiasmatic nucleus. Neurosci Lett. 2009 Sep 29; 463(1):70-3.
- Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol. 2011; 62(2):139-50.
- Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, Okamura H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997; 91(7):1043-53.
- Tanioka M, Yamada H, Doi M, Bando H, Yamaguchi Y, Nishigori C, Okamura H. Molecular clocks in mouse skin. J Invest Dermatol. 2009; 129(5):1225-31.
- Farnell YF, Shende VR, Neuendorff N, Allen GC, Earnest DJ. Immortalized cell lines for real-time analysis of circadian pacemaker and peripheral oscillator properties. Eur J Neurosci. 2011; 33(8):1533-40.
- Macchi MM, Bruce JN. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol. 2004; 25(3-4):177-95.
- Tamarkin L, Reppert SM, Klein DC, Pratt B, Goldman BD. Studies on the daily pattern of pineal melatonin in the Syrian hamster. Endocrinology. 1980; 107(5):1525-9.
- van Geijlswijk IM, Korzilius HP, Smits MG. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep. 2010; 33(12):1605-14.
- Wade AG, Ford I, Crawford G, McConnachie A, Nir T, Laudon M, Zisapel N. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 2010; 8:51.
- Lanfumey L, Mongeau R, Hamon M. Biological rhythms and melatonin in mood disorders and their treatments. Pharmacol Ther. 2013; 138(2):176-84.
- Pail G, Huf W, Pjrek E, Winkler D, Willeit M, Praschak-Rieder N, Kasper S. Bright-light therapy in the treatment of mood disorders. Neuropsychobiology. 2011; 64(3):152-62.
- Oldham MA, Ciraulo DA. Bright light therapy for depression: A review of its effects on chronobiology and the autonomic nervous system. Chronobiol Int. 2014; 31(3):305-19.
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000; 289(5488):2344-7.
- Burioka N, Takata M, Okano Y, Ohdo S, Fukuoka Y, Miyata M, Takane H, Endo M, Suyama H, Shimizu E. Dexamethasone influences human clock gene expression in bronchial epithelium and peripheral blood mononuclear cells in vitro. Chronobiol Int. 2005; 22(3):585-90.
- Nakamura TJ, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S, Shinohara K. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res. 2005; 82(5):622-30.
- Paine SJ, Gander PH, Travier N. The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30-49 years). J Biol Rhythms. 2006; 21(1):68-76.
- Beşoluk S, Onder I, Deveci I. Morningness-eveningness preferences and academic achievement of university students. Chronobiol Int. 2011; 28(2):118-25.
- Preckel F, Lipnevich AA, Boehme K, Brandner L, Georgi K, Könen T, Mursin K, Roberts RD. Morningness-eveningness and educational outcomes: the lark has an advantage over the owl at high school. Br J Educ Psychol. 2013; 83(Pt 1):114-34.
- Randler C, Kretz S. Assortative mating in morningness-eveningness. Int J Psychol. 2011; 46(2):91-6.
- Lingjaerde O, Bratlid T, Hansen T. Insomnia during the "dark period" in northern Norway. An explorative, controlled trial with light treatment.Acta Psychiatr Scand. 1985; 71(5):506-12.
- Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014; 63(6):1860-9
- Kuo SJ, Chen ST, Yeh KT, Hou MF, Chang YS, Hsu NC, Chang JG. Disturbance of circadian gene expression in breast cancer. Virchows Arch. 2009; 454(4):467-74.
- Oshima T, Takenoshita S, Akaike M, Kunisaki C, Fujii S, Nozaki A, Numata K, Shiozawa M, Rino Y, Tanaka K, Masuda M, Imada T. Expression of circadian genes correlates with liver metastasis and outcomes in colorectal cancer. Oncol Rep. 2011; 25(5):1439-46.
- Mannic T, Meyer P, Triponez F, Pusztaszeri M, Le Martelot G, Mariani O, Schmitter D, Sage D, Philippe J, Dibner C. Circadian clock characteristics are altered in human thyroid malignant nodules. J Clin Endocrinol Metab. 2013; 98(11):4446-56.
- Yeragani VK, Berger R, Pohl R, Balon R. Effect of age on diurnal changes of 24-hour QT interval variability. Pediatr Cardiol. 2005; 26(1):39-44.
- Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, Cutler MJ, Gulick J, Sanbe A, Robbins J, Demolombe S, Kondratov RV, Shea SA, Albrecht U, Wehrens XH, Rosenbaum DS, Jain MK. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012; 483(7387):96-9.
- Buijs FN, Cazarez F, Basualdo MC, Scheer FA, Perusquía M, Centurion D, Buijs RM. The suprachiasmatic nucleus is part of a neural feedback circuit adapting blood pressure response. Neuroscience. 2014; 266:197-207.
- Hahm BJ, Jo B, Dhabhar FS, Palesh O, Aldridge-Gerry A, Bajestan SN, Neri E, Nouriani B, Spiegel D, Zeitzer JM. Bedtime misalignment and progression of breast cancer. Chronobiol Int. 2014; 31(2):214-21.
- Zeng ZL, Luo HY, Yang J, Wu WJ, Chen DL, Huang P, Xu RH. Overexpression of the circadian clock gene Bmal1 increases sensitivity to oxaliplatin in colorectal cancer. Clin Cancer Res. 2014; 20(4):1042-52.
- Sato TK, Yamada RG, Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, Welsh DK, Kay SA, Ueda HR, Hogenesch JB. Feedback repression is required for mammalian circadian clock function. Nat Genet. 2006; 38(3):312-9.
- Kondratov RV, Kondratova AA, Lee C, Gorbacheva VY, Chernov MV, Antoch MP. Post-translational regulation of circadian transcriptional CLOCK(NPAS2)/BMAL1 complex by CRYPTOCHROMES. Cell Cycle. 2006; 5(8):890-5.
- Darlington TK, Wager-Smith K, Ceriani MF, Staknis D, Gekakis N, Steeves TDL, Weitz CJ, Takahashi JS, Kay SA. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science. 1998; 280:1599-603.
- McNamara P, Seo S, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001; 105:877-89.
- Langmesser S, Tallone T, Bordon A, Rusconi S, Albrecht U. Interaction of circadian clock proteins PER2 and CRY with BMAL1 and CLOCK. BMC Mol Biol. 2008; 9:41.
- Padmanabhan K, Robles MS, Westerling T, Weitz CJ. Feedback regulation of transcriptional termination by the mammalian circadian clock PERIOD complex. Science. 2012; 337:599-602.
- Busino L, Bassermann F, Maiolica A, Lee C, Nolan PM, Godinho SI, Draetta GF, Pagano M. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007; 316(5826):900-4.
- Reischl S, Vanselow K, Westermark PO, Thierfelder N, Maier B, Herzel H, Kramer A. Beta-TrCP1-mediated degradation of PERIOD2 is essential for circadian dynamics. J Biol Rhythms. 2007; 22(5):375-86.
- DeBruyne JP, Weaver DR, Reppert SM. Peripheral circadian oscillators require CLOCK. Curr Biol. 2007 Jul 17; 17(14):R538-9.
- DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007; 10(5):543-5.
- Bozek K, Relógio A, Kielbasa SM, Heine M, Dame C, Kramer A, Herzel H. Regulation of clock-controlled genes in mammals. PLoS One. 2009; 4(3):e4882.
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998; 280(5369):1564-9.
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001; 63:647-76.
- Yoo SH, Ko CH, Lowrey PL, Buhr ED, Song EJ, Chang S, Yoo OJ, Yamazaki S, Lee C, Takahashi JS. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc Acad Natl Acad Sci USA. 2005; 102:2608-13.
- Miki T, Matsumoto T, Zhao Z, Lee CC. p53 regulates Period2 expression and the circadian clock. Nat Commun. 2013; 4:2444.
- Ledent V, Vervoort M. The basic helix-loop-helix protein family: comparative genomics and phylogenetic analysis. Genome Res. 2001; 11(5):754-70.
- Murre C, McCaw PS, Vaessin H, Caudy M, Jan LY, Jan YN, Cabrera CV, Buskin JN, Hauschka SD, Lassar AB, Weintraub H, Baltimore D. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989; 58(3):537-44.
- Prendergast GC, Ziff EB. Methylation-sensitive sequence-specific DNA binding by the c-Myc basic region. Science. 1991; 251(4990):186-9.
- Zhulin IB, Taylor BL, Dixon R. PAS domain S-boxes in Archaea, Bacteria and sensors for oxygen and redox. Trends Biochem Sci. 1997; 22:331–3.
- Möglich A, Ayers RA, Moffat K. Structure and signaling mechanism of Per-ARNT-Sim domains. Structure. 2009; 17(10):1282-94.
- Henry JT, Crosson S. Ligand-binding PAS domains in a genomic, cellular, and structural context. Annu Rev Microbiol. 2011; 65:261-86.
- Pellequer JL, Wager-Smith KA, Kay SA, Getzoff ED. Photoactive yellow protein: a structural prototype for the three-dimensional fold of the PAS domain superfamily. Proc Natl Acad Sci U S A. 1998; 95:5884-90.
- Ponting CP, Aravind L. PAS: a multifunctional domain family comes to light. Curr Biol. 1997; 7(11):R674-7.
- Zhou YD, Barnard M, Tian H, Li X, Ring HZ, Francke U, Shelton J, Richardson J, Russell DW, McKnight SL. Molecular characterization of two mammalian bHLH-PAS domain proteins selectively expressed in the central nervous system. Proc. Nat. Acad. Sci. 1997; 94:713-8.
- Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997; 90:1003-11.
- Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001; 107(7):855-67.
- Kondratov RV, Chernov MV, Kondratova AA, Gorbacheva VY, Gudkov AV, Antoch MP. BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 2003; 17(15):1921-32.
- Tamanini F, Yagita K, Okamura H, van der Horst GT. Nucleocytoplasmic shuttling of clock proteins. Methods Enzymol. 2005; 393:418-35.
- Honma S, Ikeda M, Abe H, Tanahashi Y, Namihira M, Honma K, Nomura M. Circadian oscillation of BMAL1, a partner of a mammalian clock gene Clock, in rat suprachiasmatic nucleus. Biochem Biophys Res Commun. 1998; 250:83-7.
- Lincoln G, Messager S, Andersson H, Hazlerigg D. Temporal expression of seven clock genes in the suprachiasmatic nucleus and the pars tuberalis of the sheep: evidence for an internal coincidence timer. Proc Natl Acad Sci U S A. 2002; 99(21):13890-5.
- Takumi T, Matsubara C, Shigeyoshi Y, Taguchi K, Yagita K, Maebayashi Y, Sakakida Y, Okumura K, Takashima N, Okamura H. A new mammalian period gene predominantly expressed in the suprachiasmatic nucleus. Genes Cells. 1998; 3(3):167-76.
- Yan L, Takekida S, Shigeyoshi Y, Okamura H. Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience. 1999; 94(1):141-50.
- Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, Evans SJ, Choudary PV, Cartagena P, Barchas JD, Schatzberg AF, Jones EG, Myers RM, Watson SJ Jr, Akil H, Bunney WE. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A. 2013; 110(24):9950-5.
- Miyazaki K, Wakabayashi M, Chikahisa S, Sei H, Ishida N. PER2 controls circadian periods through nuclear localization in the suprachiasmatic nucleus. Genes Cells. 2007; 12(11):1225-34.
- Miyazaki K, Mesaki M, Ishida N. Nuclear entry mechanism of rat PER2 (rPER2): role of rPER2 in nuclear localization of CRY protein. Mol Cell Biol. 2001; 21(19):6651-9.
- Ukai-Tadenuma M, Yamada RG, Xu H, Ripperger JA, Liu AC, Ueda HR. Delay in feedback repression by Cryptochrome 1 is required for circadian clock function. Cell. 2011; 144:268-81.
- Miyamoto Y, Sancar A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Acad Sci U S A. 1998; 95(11):6097-102.
- Hsu DS, Zhao X, Zhao S, Kazantsev A, Wang RP, Todo T, Wei YF, Sancar A. Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry. 1996; 35:13871-7.
- Chaves I, Yagita K, Barnhoorn S, Okamura H, van der Horst GT, Tamanini F. Functional evolution of the photolyase/cryptochrome protein family: importance of the C terminus of mammalian CRY1 for circadian core oscillator performance. Mol Cell Biol. 2006; 26(5):1743-53.
- Todo T, Ryo H, Yamamoto K, Toh H, Inui T, Ayaki H, Nomura T, Ikenaga M. Similarity among the Drosophila (6-4)photolyase, a human photolyase homolog, and the DNA photolyase-blue-light photoreceptor family. Science. 1996; 272:109-12.
- van der Spek PJ, Kobayashi K, Bootsma D, Takao M, Eker APM, Yasui A. Cloning, tissue expression, and mapping of a human photolyase homolog with similarity to plant blue-light receptors. Genomics. 1996; 37:177-82.
- Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF Jr, Reppert SM. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997; 19:1261-9.
- Sangoram AM, Saez L, Antoch MP, Gekakis N, Staknis D, Whiteley A, Fruechte EM, Vitaterna MH, Shimomura K, King DP, Young MW, Weitz CJ, Takahashi JS. Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron. 1998; 21:1101-13.
- Honma S, Kawamoto T, Takagi Y, Fujimoto K, Sato F, Noshiro M, Kato Y, Honma K. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature. 2002; 419(6909):841-4.
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002; 110:251-60.
- Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004; 43:527-37.
- Unsal-Kaçmaz K, Mullen TE, Kaufmann WK, Sancar A. Coupling of human circadian and cell cycles by the timeless protein. Mol Cell Biol. 2005; 25(8):3109-16.
- Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S. A transcription factor response element for gene expression during circadian night. Nature. 2002; 418:534-9.
- Coste H, Rodríguez JC. Orphan nuclear hormone receptor Rev-erbalpha regulates the human apolipoprotein CIII promoter. J Biol Chem. 2002; 277(30):27120-9.
- Raspè E, Mautino G, Duval C, Fontaine C, Duez H, Barbier O, Monte D, Fruchart J, Fruchart JC, Staels B. Transcriptional regulation of human Rev-erbalpha gene expression by the orphan nuclear receptor retinoic acid-related orphan receptor alpha. J Biol Chem. 2002; 277(51):49275-81.
- Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nature Genet. 2005; 37:187-92.
- Crumbley C, Wang Y, Kojetin DJ, Burris TP. Characterization of the core mammalian clock component, NPAS2, as a REV-ERBalpha/RORalpha target gene. J Biol Chem. 2010; 285(46):35386-92.
- Grimaldi B, Nakahata Y, Sahar S, Kaluzova M, Gauthier D, Pham K, Patel N, Hirayama J, Sassone-Corsi P. Chromatin remodeling and circadian control: master regulator CLOCK is an enzyme. Cold Spring Harb Symp Quant Biol. 2007; 72:105-12.
- Cardone L, Hirayama J, Giordano F, Tamaru T, Palvimo JJ, Sassone-Corsi P. Circadian clock control by SUMOylation of BMAL1. Science. 2005; 309(5739):1390-4.
- Wu T, Ni Y, Kato H, Fu Z. Feeding-induced rapid resetting of the hepatic circadian clock is associated with acute induction of Per2 and Dec1 transcription in rats. Chronobiol Int. 2010; 27(1):1-18.
- Johnson CH, Golden SS, Ishiura M, Kondo T. Circadian clocks in prokaryotes. Mol Microbiol. 1996; 21:5-11.
- Dvornyk V, Deng HW, Nevo E. Structure and molecular phylogeny of sasA genes in cyanobacteria: insights into evolution of the prokaryotic circadian system. Mol Biol Evol. 2004; 21(8):1468-76.
- Ishiura M, Kutsuna S, Aoki S, Iwasaki H, Andersson CR, Tanabe A, Golden SS, Johnson CH, Kondo T. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science. 1998; 281:1519-23.
- Xu Y, Mori T, Johnson CH. Cyanobacterial circadian clockwork: roles of KaiA, KaiB and the kaiBC promoter in regulating KaiC. EMBO J. 2003; 22(9):2117-26.
- Liu Y, Tsinoremas NF, Johnson CH, Lebedeva NV, Golden SS, Ishiura M, Kondo T. Circadian orchestration of gene expression in cyanobacteria. Genes Dev. 1995; 9(12):1469-78.
- Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, Atsumi G, Ohkura N, Azama T, Mesaki M, Yukimasa S, Kobayashi H, Iitaka C, Umehara T, Horikoshi M, Kudo T, Shimizu Y, Yano M, Monden M, Machida K, Matsuda J, Horie S, Todo T, Ishida N. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem. 2003; 278(42):41519-27.
- Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993; 55:16-54.
- Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006; 22(3):375-82.
- Griniatsos J, Michail OP, Theocharis S, Arvelakis A, Papaconstantinou I, Felekouras E, Pikoulis E, Karavokyros I, Bakoyiannis C, Marinos G, Bramis J, Michail PO. Circadian variation in expression of G1 phase cyclins D1 and E and cyclin-dependent kinase inhibitors p16 and p21 in human bowel mucosa. World J Gastroenterol. 2006; 12(13):2109-14.
- Clark RH, Korst DR. Circadian periodicity of bone marrow mitotic activity and reticulocyte counts in rats and mice. Science. 1969; 166(3902):236-7.
- Smaaland R, Laerum OD, Lote K, Sletvold O, Sothern RB, Bjerknes R. DNA synthesis in human bone marrow is circadian stage dependent. Blood. 1991; 77:2603-11.
- Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chèvre R, A-González N, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, Weber C, Nagasawa T, Frenette PS, Castrillo A, Hidalgo A. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013; 153(5):1025-35.
- Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013; 341(6153):1483-8.
- Brown WR. A review and mathematical analysis of circadian rhythms in cell proliferation in mouse, rat, and human epidermis. J Invest Dermatol. 1991; 97:273-80.
- Scheving LE. Mitotic activity in the human epidermis. Anat Rec. 1959; 135:7-19.
- Hrushesky WJM, Lannin D, Haus E. Evidence for an Ontogenetic Basis for Circadian Coordination of Cancer Cell Proliferation. J Natl Cancer Inst. 1998; 90(19):1480-4.
- Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon W, Espitia F, Cam E, Millar SE, Smyth P, Ihler A, Takahashi JS, Andersen B. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc Natl Acad Sci U S A. 2012; 109(29):11758-63.
- Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Ann N Y Acad Sci. 2001; 939:200-15.
- Paulose JK, Rucker EB 3rd, Cassone VM. Toward the beginning of time: circadian rhythms in metabolism precede rhythms in clock gene expression in mouse embryonic stem cells. PLoS One. 2012; 7(11):e49555.
- O'Reilly LP, Watkins SC, Smithgall TE. An unexpected role for the clock protein timeless in developmental apoptosis. PLoS One. 2011; 6(2):e17157.
- Tsinkalovsky O, Filipski E, Rosenlund B, Sothern RB, Eiken HG, Wu MW, Claustrat B, Bayer J, Lévi F, Laerum OD. Circadian expression of clock genes in purified hematopoietic stem cells is developmentally regulated in mouse bone marrow. Exp Hematol. 2006; 34(9):1249-61.
- Spörl F, Korge S, Jürchott K, Wunderskirchner M, Schellenberg K, Heins S, Specht A, Stoll C, Klemz R, Maier B, Wenck H, Schrader A, Kunz D, Blatt T, Kramer A. Krüppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proc Natl Acad Sci U S A. 2012; 109(27):10903-8.
- Janich P, Toufighi K, Solanas G, Luis NM, Minkwitz S, Serrano L, Lehner B, Benitah SA. Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem Cell. 2013; 13(6):745-53.
- Janich P, Pascual G, Merlos-Suárez A, Batlle E, Ripperger J, Albrecht U, Cheng HY, Obrietan K, Di Croce L, Benitah SA. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011; 480(7376):209-14.
- Al-Nuaimi Y, Hardman JA, Bíró T, Haslam IS, Philpott MP, Tóth BI, Farjo N, Farjo B, Baier G, Watson RE, Grimaldi B, Kloepper JE, Paus R. A Meeting of Two Chronobiological Systems: Circadian Proteins Period1 and BMAL1 Modulate the Human Hair Cycle Clock. J Invest Dermatol. 2014; 134(3):610-9.
- Arabadjiev A, Petkova R, Momchilova A, Chakarov S, Pankov R (2012). Of mice and men – differential mechanisms of maintaining the undifferentiated state in mESC and hESC. Biodiscovery. 2012; 3:1.
- Petkova R, Arabadjiev B, Chakarov S, Rumen Pankov. Current state of the opportunities for derivation of germ-like cells from pluripotent stem cells: are you a man, or a mouse? Biotechnol & Biotechnol Eq. 2014; 28(2):184-191
- Sharma VP, Anderson NT, Geusz ME. Circadian properties of cancer stem cells in glioma cell cultures and tumorspheres. Cancer Lett. 2014; 345(1):65-74.
- Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C. Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci. 2013; 33(36):14288-300.
- Bouchard-Cannon P, Mendoza-Viveros L, Yuen A, Kærn M, Cheng HY. The circadian molecular clock regulates adult hippocampal neurogenesis by controlling the timing of cell-cycle entry and exit. Cell Rep. 2013; 5(4):961-73.
- Mueller AD, Meerlo P, McGinty D, Mistlberger RE. Sleep and Adult Neurogenesis: Implications for Cognition and Mood. Curr Top Behav Neurosci. 2013; 25: 151-181.
- Dublin WB. Absence of daily rhythm of growth of malihnant neoplasms. Northw. Med. (Seattle) 1994; 43:232.
- Voutilainen A. Uber die 24-stunden-rhythmik der mitozfrequenz in malignen tumoren. Acta Pathol Microbiol Scand. 1953; 99(suppl):1-104.
- Tahti E. Studies of the effect of x-radiation on 24-hour variations in the mitotic activity in human malignant tumors. Acta Pathol Microbiol Scand. 1956; 117(suppl):1-61.
- Klevecz RR, Shymko RM, Blumenfeld D, Braly PS. Circadian gating of S phase in human ovarian cancer. Cancer Res. 1987; 47(23):6267-71.
- Smaaland R, Lote K, Sothern R.B, Laerum OD. DNA synthesis and ploidy in non-Hodgkin's lymphomas demonstrate intrapatient variation depending on circadian stage of cell sampling. Cancer Res. 1993; 53(13):3129-38.
- Hastings JW, Sweeney BM. The action spectrum for shifting the phase of the rhythm of luminescence in Gonyaulax polyedra. J Gen Physiol. 1960; 43:697-706.
- Sweeney BM, Haxo FT, Hastings JW. Action spectra for two effects of light on luminescence in Gonyaulax polyedra. J Gen Physiol. 1959; 43:285-99.
- Sweeney BM. Resetting the Biological Clock in Gonyaulax with Ultraviolet Light. Plant Physiol. 1963; 38(6):704-8.
- Chen Z, McKnight SL. A conserved DNA damage response pathway responsible for coupling the cell division cycle to the circadian and metabolic cycles. Cell Cycle. 2007; 6(23):2906-12.
- Gamsby JJ, Loros JJ, Dunlap JC. A phylogenetically conserved DNA damage response resets the circadian clock. J Biol Rhythms. 2009; 24(3):193-202.
- Pregueiro AM, Liu Q, Baker CL, Dunlap JC, Loros JJ. The Neurospora checkpoint kinase 2: A regulatory link between the circadian and cell cycles. Science. 2006; 313:644-9.
- Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998; 282:1893-7.
- Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003; 3(5):421-9.
- Yu L, Orlandi L, Wang P, Orr MS, Senderowicz AM, Sausville EA, Silvestrini R, Watanabe N, Piwnica-Worms H, O'Connor PM. UCN-01 abrogates G2 arrest through a Cdc2-dependent pathway that is associated with inactivation of the Wee1Hu kinase and activation of the Cdc25C phosphatase. J Biol Chem. 1998; 273(50):33455-64.
- Kumagai A, Dunphy WG. Binding of 14-3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev. 1999; 13:1067-72.
- Furnari B, Blasina A, Boddy MN, McGowan CH, Russell P. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol Biol Cell. 1999; 10:833-45.
- Gardner GF, Feldman JF. Temperature Compensation of Circadian Period Length in Clock Mutants of Neurospora crassa. Plant Physiol. 1981; 68(6):1244-8.
- Lukas J, Lukas C, Bartek J. Mammalian cell cycle checkpoints: signalling pathways and their organization in space and time. DNA Repair (Amst). 2004; 3(8-9):997-1007.
- Chen-Goodspeed M, Lee CC. Tumor suppression and circadian function. J Biol Rhythms. 2007; 22(4):291-8.
- Lee CH, Chung JH. The hCds1 (Chk2)-FHA domain is essential for a chain of phosphorylation events on hCds1 that is induced by ionising radiation. J Biol Chem. 2001; 276(32):30537-41.
- Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G-1 by stabilising p53. Genes Dev. 2000; 14:278-88.
- Tang J, Erikson RL, Liu X. Checkpoint kinase 1 (Chk1) is required for mitotic progression through negative regulation of polo-like kinase 1 (Plk1). Proc Nat Acad Sci USA. 2006; 103:11964-9.
- Yang X, Wood PA, Hrushesky WJ. Mammalian TIMELESS is required for ATM-dependent CHK2 activation and G2/M checkpoint control. J Biol Chem. 2010; 285(5):3030-4.
- Zhang EE, Liu AC, Hirota T, Miraglia LJ, Welch G, Pongsawakul PY, Liu X, Atwood A, Huss JW 3rd, Janes J, Su AI, Hogenesch JB, Kay SA. A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell. 2009; 139(1):199-210.
- Sancar A, Lindsey-Boltz LA, Kang TH, Reardon JT, Lee JH, Ozturk N. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 2010; 584(12):2618-25.
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002; 111(1):41-50.
- Chakarov S, Petkova R, Russev GC. p53 – guardian angel and archangel. Biotechnol. Biotechnol. Eq. 2012; 26(1):2695-702.
- Mullenders J, Fabius A.W, Madiredjo M, Bernards R, Beijersbergen RL. A large scale shRNA barcode screen identifies the circadian clock component ARNTL as putative regulator of the p53 tumor suppressor pathway. PLoS One. 2009; 4(3):e4798.
- Ozturk N, Lee JH, Gaddameedhi S, Sancar A. Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc Natl Acad Sci U S A. 2009; 106(8):2841-6.
- Walhout AJ, Gubbels JM, Bernards R, van der Vliet PC, Timmers HT. c-Myc/Max heterodimers bind cooperatively to the E-box sequences located in the first intron of the rat ornithine decarboxylase (ODC) gene. Nucleic Acids Res. 1997; 25(8):1493-501.
- Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer. 2003; 3(5):350-61.
- Lee CC. The circadian clock and tumor suppression by mammalian period genes. Methods Enzymol. 2005; 393:852-61.
- Bhawal UK, Sato F, Arakawa Y, Fujimoto K, Kawamoto T, Tanimoto K, Ito Y, Sasahira T, Sakurai T, Kobayashi M, Kashima I, Kijima H, Kuniyasu H, Abiko Y, Kato Y, Sato S. Basic helix-loop-helix transcription factor DEC1 negatively regulates cyclin D1. J Pathol. 2011; 224(3):420-9.
- Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003; 302(5643):255-9.
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999; 98(2):193-205.
- Kang TH, Reardon JT, Kemp M, Sancar A. Circadian oscillation of nucleotide excision repair in mammalian brain. Proc Natl Acad Sci USA. 2009; 106(8):2864-7.
- Gaddameedhi S, Reardon JT, Ye R, Ozturk N, Sancar A. Effect of circadian clock mutations on DNA damage response in mammalian cells. Cell Cycle. 2012; 11(18):3481-91.
- Kang TH, Lindsey-Boltz LA, Reardon JT, Sancar A. Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc Natl Acad Sci USA. 2010; 107(11):4890-5.
- Marchenay C, Cellarier E, Lévi F, Rolhion C, Kwiatkowski F, Claustrat B, Madelmont JC, Chollet P. Circadian variation in O6-alkylguanine-DNA alkyltransferase activity in circulating blood mononuclear cells of healthy human subjects. Int J Cancer. 2001; 91(1):60-6.
- Kim J, Matsunaga N, Koyanagi S, Ohdo S. Clock gene mutation modulates the cellular sensitivity to genotoxic stress through altering the expression of N-methylpurine DNA glycosylase gene. Biochem Pharmacol. 2009; 78(8):1075-82.
- Grimaldi B, Nakahata Y, Kaluzova M, Masubuchi S, Sassone-Corsi P. Chromatin remodeling, metabolism and circadian clocks: the interplay of CLOCK and SIRT1. Int J Biochem Cell Biol. 2009; 41(1):81-6.
- Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007; 450(7172):1086-90.
- Hoppe G, Rayborn M.E, Sears JE. Diurnal rhythm of the chromatin protein Hmgb1 in rat photoreceptors is under circadian regulation. J Comp Neurol. 2007; 501(2):219-30.
- Wang Z, Zang C, Rosenfeld J.A, Schones D.E, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008; 40(7):897-903.
- Ishihara H, Tanaka I, Yakumaru H, Chikamori M, Ishihara F, Tanaka M, Ishiwata A, Kurematsu A, Satoh A, Ueda J, Akashi M. Circadian transitions in radiation dose-dependent augmentation of mRNA levels for DNA damage-induced genes elicited by accurate real-time RT-PCR quantification. J Radiat Res. 2010; 51(3):265-75.
- Duffy JF, Wright KP Jr. Entrainment of the human circadian system by light. J Biol Rhythms. 2005; 20(4):326-38.
- Wright KP Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005; 20(2):168-77.
- Mellon I, Bhor VA, Hanawalt PC. Preferential repair of an active gene in human cells. Proc Natl Acad Sci USA. 1986; 83:8878-82.
- Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY). 2011; 3(5):479-93.
- Rensing L, Meyer-Grahle U, Ruoff P. Biological timing and the clock metaphor: oscillatory and hourglass mechanisms. Chronobiol Int. 2001; 18(3):329-69.
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000; 103(7):1009-17.
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006; 20(14):1868-73.
- Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz J.M, Williams C Moss S, Sehgal A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008; 23(1):26-36.
- Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One. 2010; 5:e109
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna M.H, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi J.S, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010; 466(7306):627-31.
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995; 82(4):675-84.
- Leliavski A, Shostak A, Husse J, Oster H. Impaired glucocorticoid production and response to stress in Arntl-deficient male mice. Endocrinology. 2014 Jan; 155(1):133-42.
- Feillet CA, Albrecht U, Challet EJ. "Feeding time" for the brain: a matter of clocks. Physiol Paris. 2006; 100(5-6):252-60.
- Wu X, Wiater MF, Ritter S. NPAS2 deletion impairs responses to restricted feeding but not to metabolic challenges. Physiol Behav. 2010 Mar 30; 99(4):466-71.
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TDL, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian Clock gene. Cell. 1997; 89:641-53.
- Wakatsuki Y, Kudo T, Shibata S. Constant light housing during nursing causes human DSPS (delayed sleep phase syndrome) behaviour in Clock-mutant mice. Eur J Neurosci. 2007; 25(8):2413-24.
- Kennaway DJ, Boden MJ, Voultsios A. Reproductive performance in female clock Delta19 mutant mice. Reprod Fertil Dev. 2004; 16:801-10.
- Dolatshad H, Campbell E.A, O'Hara L, Maywood ES, Hastings MH, Johnson MH. Developmental and reproductive performance in circadian mutant mice. Hum Reprod. 2006; 21:68-79.
- Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY). 2010; 2(12):936-44.
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005; 308(5724):1043-5.
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DI, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Nat Acad Sci USA. 2005; 102:9377-81.
- van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, Buijs R, Bootsma D, Hoeijmakers JH, Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999; 398(6728):627-30.
- Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, Takahashi JS, Sancar A. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci USA. 1999; 96(21):12114-9.
- Shearman LP, Jin X, Lee C, Reppert SM, Weaver DR. Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Molec Cell Biol. 2000; 20:6269-75.
- Barlow C, Eckhaus MA, Schäffer AA, Wynshaw-Boris A. Atm haploinsufficiency results in increased sensitivity to sublethal doses of ionizing radiation in mice. Nat Genet. 1999; 21(4):359-60.
- Pilorz V, Steinlechner S. Low reproductive success in Per1 and Per2 mutant mice females due to accelerated ageing? Reproduction. 2008; 135:559-68.
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitisation and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002; 99(13):9026-30.
- Filipski E, Innominato PF, Wu M, Li XM, Iacobelli S, Xian LJ, Levi F. Effects of light and food schedules on liver and tumor molecular clocks in mice. J Natl Cancer Inst. 2005; 97:507-17.
- Harvey KJ, Lukovic D, Ucker DS. Caspase-dependent Cdk activity is a requisite effector of apoptotic death events. J Cell Biol. 2000; 148(1):59-72.
- Adachi S, Obaya AJ, Han Z, Ramos-Desimone N, Wyche JH, Sedivy JM.c-Myc is necessary for DNA damage-induced apoptosis in the G(2) phase of the cell cycle. Mol Cell Biol. 2001; 21(15):4929-37.
- Winter SL, Bosnoyan-Collins L, Pinnaduwage D, Andrulis IL. Expression of the circadian clock genes Per1 and Per2 in sporadic and familial breast tumors. Neoplasia. 2007; 9(10):797-800.
- Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ, Fu Y-H. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001; 291(5506):1040-3.
- He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL Jr, Rossner MJ, Nishino S, Fu Y-H. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009; 325(5942):866-70.
- Katzenberg D, Young T, Finn L, Lin L, King DP, Takahashi JS, Mignot E. A clock polymorphism associated with human diurnal preference. Sleep. 1998; 21:569-76.
- Mishima K, Tozawa T, Satoh K, Saitoh H, Mishima Y. The 3111T/C polymorphism of hClock is associated with evening preference and delayed sleep timing in a Japanese population sample. Am J Med Genet B Neuropsychiatr Genet. 2005; 133:101-4.
- Robilliard DL, Archer SN, Arendt J, Lockley SW, Hack LM, English J, Leger D, Smits MG, Williams A, Skene DJ, von Schantz M. The 3111 clock gene polymorphism is not associated with sleep and circadian rhythmicity in phenotypically characterized human subjects. Journal of Sleep Research. 2002; 11:305-12.
- Chang AM, Buch AM, Bradstreet DS, Klements DJ, Duffy JF. Human diurnal preference and circadian rhythmicity are not associated with the CLOCK 3111C/T gene polymorphism. J Biol Rhythms. 2011; 26(3):276-9.
- Kissling C, Retz W, Wiemann S, Coogan AN, Clement RM, Hünnerkopf R, Conner AC, Freitag CM, Rösler M, Thome J. A polymorphism at the 3'-untranslated region of the CLOCK gene is associated with adult attention-deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008; 147(3):333-8.
- Soria V, Martínez-Amorós E, Escaramís G, Valero J, Pérez-Egea R, García C, Gutiérrez-Zotes A, Puigdemont D, Bayés M, Crespo JM, Martorell L, Vilella E, Labad A, Vallejo J, Pérez V, Menchón JM, Estivill X, Gratacòs M, Urretavizcaya M. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology. 2010; 35(6):1279-89.
- Hua P, Liu W, Chen D, Zhao Y, Chen L, Zhang N, Wang C, Guo S, Wang L, Xiao H, Kuo SH. Cry1 and Tef gene polymorphisms are associated with major depressive disorder in the Chinese population. J Affect Disord. 2014; 157:100-3.
- Garaulet M, Smith CE, Gomez-Abellán P, Ordovás-Montañés M, Lee YC, Parnell LD, Arnett DK, Ordovás JM. REV-ERB-ALPHA circadian gene variant associates with obesity in two independent populations: Mediterranean and North American. Mol Nutr Food Res. 2014; 58(4):821-9.
- Karantanos T, Theodoropoulos G, Gazouli M, Vaiopoulou A, Karantanou C, Stravopodis DJ, Bramis K, Lymperi M, Pektasidis D. Association of the clock genes polymorphisms with colorectal cancer susceptibility. J Surg Oncol. 2013; 108(8):563-7.
- Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, von Schantz M. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003; 26(4):413-5.
- Zhu Y, Brown HN, Zhang Y, Stevens RG, Zheng T. Period3 structural variation: a circadian biomarker associated with breast cancer in young women. Cancer Epidemiol Biomarkers Prev. 2005; 14(1):268-70.
- Zhu Y, Leaderer D, Guss C, Brown HN, Zhang Y, Boyle P, Stevens RG, Hoffman A, Qin Q, Han X, Zheng T. Ala394Thr polymorphism in the clock gene NPAS2: a circadian modifier for the risk of non-Hodgkin's lymphoma. Int J Cancer. 2007; 120(2):432-5.
- Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology. 2006; 17(1):108-11.
- Stevens RG. Artificial lighting in the industrialized world: circadian disruption and breast cancer. Cancer Causes Control. 2006; 17(4):501-7.
- Hansen J, Lassen CF. Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med. 2012; 69(8):551-6.
- Flynn-Evans EE, Mucci L, Stevens RG, Lockley SW. Shiftwork and prostate-specific antigen in the National Health and Nutrition Examination Survey. J Natl Cancer Inst. 2013; 105(17):1292-7.
- Schernhammer ES, Feskanich D, Liang G, Han J. Rotating night-shift work and lung cancer risk among female nurses in the United States. Am J Epidemiol. 2013 Nov 1; 178(9):1434-41.
- Sigurdardottir LG, Valdimarsdottir UA, Mucci LA, Fall K, Rider JR, Schernhammer E, Czeisler CA, Launer L, Harris T, Stampfer MJ, Gudnason V, Lockley SW. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2013; 22(5):872-9.
- Feychting M, Osterlund B, Ahlbom A. Reduced cancer incidence among the blind. Epidemiology. 1998; 9(5):490-4.
- Kliukiene J, Tynes T, Andersen A. Risk of breast cancer among Norwegian women with visual impairment. Br J Cancer. 2001; 84(3):397-9.
- Flynn-Evans EE, Stevens RG, Tabandeh H, Schernhammer ES, Lockley S. Total visual blindness is protective against breast cancer. Cancer Causes Control. 2009; 20(9):1753-6.
- Pukkala E, Ojamo M, Rudanko SL, Stevens RG, Verkasalo PK. Does incidence of breast cancer and prostate cancer decrease with increasing degree of visual impairment? Cancer Causes Control. 2006; 17(4):573-6.
- Savvidis C, Koutsilieris M. Circadian rhythm disruption in cancer biology. Mol Med. 2012; 18:1249-60.
- Schwimmer H, Metzer A, Pilosof Y, Szyf M, Machnes ZM, Fares F, Harel O, Haim A. Light at night and melatonin have opposite effects on breast cancer tumors in mice assessed by growth rates and global DNA methylation. Chronobiol Int. 2014; 31(1):144-50.
- Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Gréchez-Cassiau A, Guettier C, Hastings MH, Francis L. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004; 64(21):7879-85.
- Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, Lellouch J, Misset JL, Touitou Y, Lévi F. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000; 6(8):3038-45.
- Innominato PF, Giacchetti S, Bjarnason GA, Focan C, Garufi C, Coudert B, Iacobelli S, Tampellini M, Durando X, Mormont MC, Waterhouse J, Lévi FA. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. Int J Cancer. 2012; 131(11):2684-92.
- Mormont MC, Langouët AM, Claustrat B, Bogdan A, Marion S, Waterhouse J, Touitou Y, Lévi F. Marker rhythms of circadian system function: a study of patients with metastatic colorectal cancer and good performance status. Chronobiol Int. 2002; 19(1):141-55.
- Hsu CM, Lin SF, Lu CT, Lin PM, Yang MY. Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumour Biol. 2012; 33(1):149-55.
- Mazzoccoli G, Piepoli A, Carella M, Panza A, Pazienza V, Benegiamo G, Palumbo O, Ranieri E. Altered expression of the clock gene machinery in kidney cancer patients. Biomed Pharmacother. 2012; 66(3):175-9.
- Relles D, Sendecki J, Chipitsyna G, Hyslop T, Yeo CJ, Arafat HA. Circadian gene expression and clinicopathologic correlates in pancreatic cancer. J Gastrointest Surg. 2013; 17(3):443-50.
- Zhao H, Zeng ZL, Yang J, Jin Y, Qiu MZ, Hu XY, Han J, Liu KY, Liao JW, Xu RH, Zou QF. Prognostic relevance of Period1 (Per1) and Period2 (Per2) expression in human gastric cancer. Int J Clin Exp Pathol. 2014; 7(2):619-30.
- Yang X, Wood PA, Oh EY, Du-Quiton J, Ansell CM, Hrushesky WJ. Down regulation of circadian clock gene Period 2 accelerates breast cancer growth by altering its daily growth rhythm. Breast Cancer Res Treat. 2009; 117(2):423-31.
- Miyazaki K, Wakabayashi M, Hara Y, Ishida N. Tumor growth suppression in vivo by overexpression of the circadian component, PER2. Genes Cells. 2010; 15(4):351-8.
- Nishiwaki T, Daigo Y, Kawasoe T, Nakamura Y. Isolation and mutational analysis of a novel human cDNA, DEC1 (deleted in esophageal cancer 1), derived from the tumor suppressor locus in 9q32. Genes Chromosomes Cancer. 2000; 27:169-76.
- Eisele L, Prinz R, Klein-Hitpass L, Nückel H, Lowinski K, Thomale J, Moeller LC, Dührsen U, Dürig J. Combined PER2 and CRY1 expression predicts outcome in chronic lymphocytic leukemia. Eur J Haematol. 2009; 83(4):320-7.
- Hanoun M, Eisele L, Suzuki M, Greally JM, Hüttmann A, Aydin S, Scholtysik R, Klein-Hitpass L, Dührsen U, Dürig J. Epigenetic silencing of the circadian clock gene CRY1 is associated with an indolent clinical course in chronic lymphocytic leukemia. PLoS One. 2012; 7(3):e34347.
- Rana S, Munawar M, Shahid A, Malik M, Ullah H, Fatima W, Mohsin S, Mahmood S. Deregulated expression of circadian clock and clock-controlled cell cycle genes in chronic lymphocytic leukemia. Mol Biol Rep. 2014; 41(1):95-103.
- Yu H, Meng X, Wu J, Pan C, Ying X, Zhou Y, Liu R, Huang W. Cryptochrome 1 overexpression correlates with tumor progression and poor prognosis in patients with colorectal cancer. PLoS One. 2013; 8(4):e61679.
- Bernard S, Cajavec Bernard B, Lévi F, Herzel H. Tumor growth rate determines the timing of optimal chronomodulated treatment schedules. PLoS Comput Biol. 2010 Mar 19; 6(3):e1000712.
- Yang X, Wood PA, Ansell CM, Quiton DF, Oh EY, Du-Quiton J, Hrushesky WJ. The circadian clock gene Per1 suppresses cancer cell proliferation and tumor growth at specific times of day. Chronobiol Int. 2009; 26(7):1323-39.
- Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: A novel association. Physiol Behav. 2014; 134:44-50.
- de Oliveira C, Scarabelot VL, de Souza A, de Oliveira CM, Medeiros LF, de Macedo IC, Marques Filho PR, Cioato SG, Caumo W, Torres IL. Obesity and chronic stress are able to desynchronize the temporal pattern of serum levels of leptin and triglycerides. Peptides. 2014 Jan; 51:46-53.
- Shamsi NA, Salkeld MD, Rattanatray L, Voultsios A, Varcoe TJ, Boden MJ, Kennaway DJ. Metabolic consequences of timed feeding in mice. Physiol Behav. 2014 Apr 10; 128:188-201.
- Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J Biol Rhythms. 2011; 26(5):423-33.
- Rakshit K, Thomas AP, Matveyenko AV. Does disruption of circadian rhythms contribute to Beta-cell failure in type 2 diabetes? Curr Diab Rep. 2014; 14(4):474.
- Mendoza J, Pevet P, Challet E. High-fat feeding alters the clock synchronization to light. J. Physiol. 2008; 586:5901-10.
- Pendergast JS, Branecky KL, Huang R, Niswender KD, Yamazaki S. Wheel-running activity modulates circadian organization and the daily rhythm of eating behavior. Front Psychol. 2014; 5:177.
- Hwang JW, Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J. 2014; 28(1):176-94.
- Fujii T, Uzu T, Nishimura M, Takeji M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Circadian rhythm of natriuresis is disturbed in nondipper type of essential hypertension. Am J Kidney Dis. 1999; 33(1):29-35.
- Kimura G, Dohi Y, Fukuda M. Salt sensitivity and circadian rhythm of blood pressure: the keys to connect CKD with cardiovascular events. Hypertens Res. 2010; 33(6):515-20.
- Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998; 29(5):992-6.
- Willich SN. Circadian variation and triggering of cardiovascular events. Vasc Med. 1999; 4(1):41-9.
- Karmarkar SW, Tischkau SA. Influences of the circadian clock on neuronal susceptibility to excitotoxicity. Front Physiol. 2013; 4:313.
- Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007 Jul; 130(Pt 7):1873-83.
- Sumpter RE, Dorris L, Kelly T, McMillan TM. Pediatric sleep difficulties after moderate-severe traumatic brain injury. J Int Neuropsychol Soc. 2013; 19(7):829-34.
- Boone DR, Sell SL, Micci MA, Crookshanks JM, Parsley M, Uchida T, Prough DS, DeWitt DS, Hellmich HL Traumatic brain injury-induced dysregulation of the circadian clock. PLoS One. 2012; 7(10):e46204.
- Tischkau SA, Cohen JA, Stark JT, Gross DR, Bottum KM. Time-of-day affects expression of hippocampal markers for ischemic damage induced by global ischemia. Exp Neurol. 2007; 208(2):314-22.
- Cavalcanti PR, Campos TF, Araüjo JF. Circadian and homeostatic changes of sleep-wake and quality of life in stroke: implications for neurorehabilitation. NeuroRehabilitation. 2013; 32(2):337-43.
- Wiebking N, Maronde E, Rami A. Increased neuronal injury in clock gene Per-1 deficient-mice after cerebral ischemia. Curr Neurovasc Res. 2013; 10(2):112-25.
- Uth K, Sleigh R. Deregulation of the circadian clock constitutes a significant factor in tumorigenesis: a clockwork cancer. Part II: In vivo studies. Biotechnol & Biotechnol. Eq. 2014; 28(3):379-386.
- Gögenur I, Ocak U, Altunpinar O, Middleton B, Skene DJ, Rosenberg J. Disturbances in melatonin, cortisol and core body temperature rhythms after major surgery. World J Surg. 2007; 31(2):290-8.
- Gögenur I. Postoperative circadian disturbances. Dan Med Bull. 2010 Dec; 57(12):B4205.
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007; 114:222-32.
- Hayes PE, Ettigi P. Dexamethasone suppression test in diagnosis of depressive illness. Clin Pharm. 1983; 2(6):538-45.
- Rush AJ, Giles DE, Schlesser MA, Orsulak PJ, Parker CR Jr, Weissenburger JE, Crowley GT, Khatami M, Vasavada N. The dexamethasone suppression test in patients with mood disorders. J Clin Psychiatry. 1996; 57(10):470-84.
- Keller J, Flores B, Gomez RG, Solvason HB, Kenna H, Williams GH, Schatzberg AF. Cortisol circadian rhythm alterations in psychotic major depression. Biol Psychiatry. 2006; 60(3):275-81.
- Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, Edwards RA, Sahar S, Dandekar S, Baldi P, George MD, Raffatellu M, Sassone-Corsi P. Circadian clock regulates the host response to Salmonella. Proc Natl Acad Sci U S A. 2013; 110(24):9897-902.
- Kunieda T, Minamino T, Katsuno T, Tateno K, Nishi J, Miyauchi H, Orimo M, Okada S, Komuro I. Cellular senescence impairs circadian expression of clock genes in vitro and in vivo. Circ Res. 2006; 98(4):532-9.
- Qu Y, Mao M, Li X, Liu Y, Ding J, Jiang Z, Wan C, Zhang L, Wang Z, Mu D. Telomerase reconstitution contributes to resetting of circadian rhythm in fibroblasts. Mol Cell Biochem. 2008; 313(1-2):11-8.
- Agirbasli M. Pivotal role of plasminogen-activator inhibitor 1 in vascular disease. Int J Clin Pract. 2005; 59(1):102-6.
- Testa U, Pannitteri G, Condorelli GL. Vascular endothelial growth factors in cardiovascular medicine. J Cardiovasc Med (Hagerstown). 2008; 9(12):1190-221.
- Maemura K, de la Monte SM, Chin MT, Layne MD, Hsieh CM, Yet SF, Perrella MA, Lee ME. CLIF, a novel cycle-like factor, regulates the circadian oscillation of plasminogen activator inhibitor-1 gene expression. J Biol Chem. 2000; 275:36847-51.
- Koyanagi S, Kuramoto Y, Nakagawa H, Aramaki H, Ohdo S, Soeda S, Shimeno H. A molecular mechanism regulating circadian expression of vascular endothelial growth factor in tumor cells. Cancer Res. 2003; 63:7277-83.
- Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013; 153(1):13-9.
- Ferroni P, Roselli M, Portarena I, Formica V, Riondino S, LA Farina F, Costarelli L, Melino A, Massimiani G, Cavaliere F, Palmirotta R, Guadagni F. Plasma plasminogen activator inhibitor-1 (PAI-1) levels in breast cancer - relationship with clinical outcome. Anticancer Res. 2014; 34(3):1153-61.
- Skene DJ, Vivien-Roels B, Sparks DL, Hunsaker JC, Pevet P, Ravid D, Swaab DF. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: effect of age and Alzheimer's disease. Brain Res. 1990; 528:170-4.
- Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer's dementia. Brain. 2004; 127(Pt 5):1061-74.
- Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, Liu RY, Zhou JN, Swaab DF. Pineal clock gene oscillation is disturbed in Alzheimer's disease, due to functional disconnection from the master clock. FASEB J. 2006; 20(11):1874-6.
- Wu YH, Swaab DF. The human pineal gland and melatonin in aging and Alzheimer's disease. J Pineal Res. 2005; 38(3):145-52.
- Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, Rademaker AW, Simuni T, Zadikoff C, Zee PC. Circadian Melatonin Rhythm and Excessive Daytime Sleepiness in Parkinson Disease. JAMA Neurol. 2014; 71(4):463-9.
- Khalil HS, Petkova R, Zhelev N. Differential genetic advantages in youth and in aging, or how to die healthy. Biotechnol Biotechnol Eq. 2012; 26(1):2703-11.
- Arnold J, Dai J, Nahapetyan L, Arte A, Johnson MA, Hausman D, Rodgers WL, Hensley R, Martin P, Macdonald M, Davey A, Siegler IC, Jazwinski SM, Poon LW. Predicting successful aging in a population-based sample of georgia centenarians. Curr Gerontol Geriatr Res. 2010; 2010:989315.
- Froy O. The relationship between nutrition and circadian rhythms in mammals. Front Neuroendocrinol. 2007; 28(2-3):61-71.
- Navas-Carretero S, Abete I Zulet MA, Martínez JA. Chronologically scheduled snacking with high-protein products within the habitual diet in type-2 diabetes patients leads to a fat mass loss: a longitudinal study. Nutr J. 2012; 10:74.
- Kagawa Y. Chronological nutrition and prevention of lifestyle-related diseases. Nihon Rinsho. 2012; 70(7):1233-40.
- Tampellini M, Filipski E, Liu XH, Lemaigre G, Li XM, Vrignaud P, François E, Bissery MC, Lévi F. Docetaxel chronopharmacology in mice. Cancer Res. 1998; 58(17):3896-904.
- Granda TG, Lévi F. Tumor-based rhythms of anticancer efficacy in experimental models. Chronobiol Int. 2002; 19:21-41.
- Granda TG, D'Attino RM, Filipski E, Vrignaud P, Garufi C, Terzoli E, Bissery MC, Lévi F. Circadian optimisation of irinotecan and oxaliplatin efficacy in mice with Glasgow osteosarcoma. Br J Cancer. 2002; 86(6):999-1005.
- Halberg F, Haus E, Cardoso SS, Scheving LE, Kühl JF, Shiotsuka R, Rosene G, Pauly JE, Runge W, Spalding JF, Lee JK, Good RA. Toward a chronotherapy of neoplasia: tolerance of treatment depends upon host rhythms. Experientia. 1973; 29(8):909-34.
- Wilson WL. Iatrogenic toxicity from cancer chemotherapy-can it be reduced by timing treatment according to rhythms? Int J Chronobiol. 1974; 2(2):171-3.
- Focan C. Sequential chemotherapy and circadian rhythm in human solid tumours. A randomised trial. Cancer Chemother Pharmacol. 1979; 3(3):197-202.
- Hrushesky WJ. Circadian timing of cancer chemotherapy. Science. 1985; 228:73-5.
- Lévi FA, Zidani R, Vannetzel JM, Perpoint B, Focan C, Faggiuolo R, Chollet P, Garufi C, Itzhaki M, Dogliotti L. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst. 1994; 86(21):1608-17.
- Bertheault-Cvitkovic F, Jami A, Ithzaki M, Brummer PD, Brienza S, Adam R, Kunstlinger F, Bismuth H, Misset JL, Lévi F. Biweekly intensified ambulatory chronomodulated chemotherapy with oxaliplatin, fluorouracil, and leucovorin in patients with metastatic colorectal cancer. J Clin Oncol. 1996; 14(11):2950-8.
- Curé H, Chevalier V, Adenis A, Tubiana-Mathieu N, Niezgodzki G, Kwiatkowski F, Pezet D, Perpoint B, Coudert B, Focan C, Lévi F, Chipponi J, Chollet P. Phase II trial of chronomodulated infusion of high-dose fluorouracil and l-folinic acid in previously untreated patients with metastatic colorectal cancer. J Clin Oncol. 2002; 20(5):1175-81.
- Focan C, Demolin G, Kreutz F, Graas MP, Longrée L, Matus G, Moeneclaey N, Focan-Henrard D. Chronotherapy with 5-fluorouracil folinic acid and oxaliplatin delivered over 48 hours every second week in colorectal cancer. The CHC-Liège experience (Belgium). Pathol Biol (Paris). 2013; 61(5):e71-4.
- Filipski E, Berland E, Ozturk N, Guettier C, van der Horst GT, Lévi F, Okyar A. Optimization of irinotecan chronotherapy with P-glycoprotein inhibition. Toxicol Appl Pharmacol. 2014; 274(3):471-9.
- Giacchetti S, Bjarnason G, Garufi C, Genet D, Iacobelli S, Tampellini M, Smaaland R, Focan C, Coudert B, Humblet Y, Canon JL, Adenis A, Lo Re G, Carvalho C, Schueller J, Anciaux N, Lentz MA, Baron B, Gorlia T, Lévi F; European Organisation for Research and Treatment of Cancer Chronotherapy Group. Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for Research and Treatment of Cancer Chronotherapy Group. J Clin Oncol. 2006; 24(22):3562-9.
- Innominato PF, Giacchetti S, Moreau T, Smaaland R, Focan C, Bjarnason GA, Garufi C, Iacobelli S, Tampellini M, Tumolo S, Carvalho C, Karaboué A, Lévi F; ARTBC International Chronotherapy Group. Prediction of survival by neutropenia according to delivery schedule of oxaliplatin-5-Fluorouracil-leucovorin for metastatic colorectal cancer in a randomized international trial (EORTC 05963). Chronobiol Int. 2011; 28(7):586-600.
- Innominato PF, Giacchetti S, Moreau T, Bjarnason GA, Smaaland R, Focan C, Garufi C, Iacobelli S, Tampellini M, Tumolo S, Carvalho C, Karaboué A, Poncet A, Spiegel D, Lévi F; International Association for Research on Time in Biology and Chronotherapy (ARTBC) Chronotherapy Group. Fatigue and weight loss predict survival on circadian chemotherapy for metastatic colorectal cancer. Cancer. 2013; 119(14):2564-73.
- Giacchetti S, Dugué PA, Innominato PF, Bjarnason GA, Focan C, Garufi C, Tumolo S, Coudert B, Iacobelli S, Smaaland R, Tampellini M, Adam R, Moreau T, Lévi F; ARTBC International Chronotherapy Group. Sex moderates circadian chemotherapy effects on survival of patients with metastatic colorectal cancer: a meta-analysis. Ann Oncol. 2012; 23(12):3110-6.
- Khalil HS, Tummala H, Chakarov S, Zhelev N, Lane DP. Targeting ATM pathway for therapeutic intervention in cancer. Biodiscovery. 2012; 1:3.
- Idowu MA, Khalil HS, Bown JL, Zhelev N. Reverse engineering of drug induced DNA damage response signalling pathway reveals dual outcomes of ATM kinase inhibition. Biodiscovery. 2013; 9:4.
- Kang HS, Angers M, Beak JY, Wu X, Gimble JM, Wada T, Xie W, Collins JB, Grissom SF, Jetten AM. Gene expression profiling reveals a regulatory role for ROR α and ROR γ in phase I and phase II metabolism. Physiol Genomics. 2007; 31:281-94.
- Lau P, Fitzsimmons RL, Raichur S, Wang SC, Lechtken A, Muscat GE. The orphan nuclear receptor, RORalpha, regulates gene expression that controls lipid metabolism: staggerer (SG/SG) mice are resistant to diet-induced obesity. J Biol Chem. 2008; 283:18411-21.
- Jetten AM, Ueda E. Retinoid-related orphan receptors (RORs): roles in cell survival, differentiation and disease. Cell Death Differ. 2002; 9:1167-71.
- Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009; 7:e003.
- Vu-Dac N, Gervois P, Grotzinger T, De Vos P, Schoonjans K, Fruchart JC, Auwerx J, Mariani J, Tedgui A, Staels B. Transcriptional regulation of apolipoprotein A-I gene expression by the nuclear receptor RORalpha. J Biol Chem. 1997; 272:22401-4.
- Raspè E, Duez H, Gervois P, Fievet C, Fruchart JC, Besnard S, Mariani J, Tedgui A, Staels B. Transcriptional regulation of apolipoprotein C-III gene expression by the orphan nuclear receptor RORalpha. J Biol Chem. 2001; 276:2865-71.
- Genoux A, Dehondt H, Helleboid-Chapman A, Duhem C, Hum DW, Martin G, Pennacchio LA, Staels B, Fruchart-Najib J, Fruchart JC. Transcriptional regulation of apolipoprotein A5 gene expression by the nuclear receptor RORalpha. Arterioscler Thromb Vasc Biol. 2005; 25:1186-92.
- Zhu Y, McAvoy S, Kuhn R, Smith DI. RORA, a large common fragile site gene, is involved in cellular stress response. Oncogene. 2006; 25:2901-8.
- Lu Y, Yi Y, Liu P, Wen W, James M, Wang D, You M. Common human cancer genes discovered by integrated gene-expression analysis. PLoS ONE. 2007; 2:e1149.
- Ueda E, Kurebayashi S, Sakaue M, Backlund M, Koller B, Jetten AM. High incidence of T-cell lymphomas in mice deficient in the retinoid-related orphan receptor RORgamma. Cancer Res. 2002; 62:901-9.
- Jetten AM. Recent advances in the mechanisms of action and physiological functions of the retinoid-related orphan receptors (RORs). Curr Drug Targets Inflamm Allergy. 2004; 3(4):395-412.
- Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov. 2014; 13(3):197-216.