|
BioDiscovery : Research Article
|
|
Corresponding author: Madalene Heng (madalene@madalenehengmd.com)
Academic editor: Nikolai Zhelev
Received: 15 Nov 2016 | Accepted: 02 Feb 2017 | Published: 24 Feb 2017
© 2017 Madalene Heng
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Heng M (2017) Phosphorylase Kinase Inhibition Therapy in Burns and Scalds. BioDiscovery 20: e11207. https://doi.org/10.3897/biodiscovery.20.e11207
|
|
Abstract
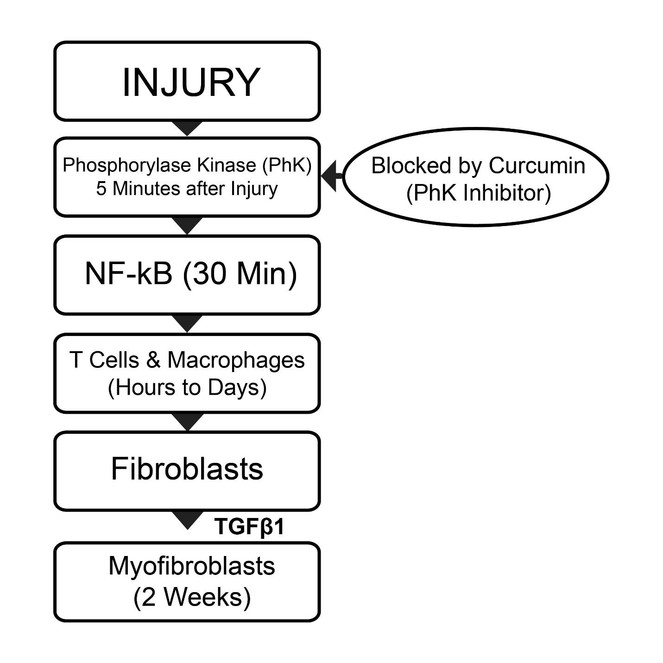
Severe burns and scalds almost always result in unsightly hypertrophic scarring. Among the important processes involved in scarring are fibroblast formation and transformation of fibroblasts into myofibroblasts. Myofibroblasts contain α-smooth muscle actin which has contractile properties and can lead to wound contraction and hypertrophic scarring. Phosphorylase kinase (PhK), expressed within 5 mins of injury, is among the earliest enzymes released after tissue damage. It is responsible for activation of NF-kB, which in turn activates over 200 different genes related to inflammation, fibroblastic proliferation, myofibroblast conversion, and eventual scar tissue formation. The sequence and approximate timing of events following injury include the following: activation of PhK (5 mins), followed by appearance of neutrophils (30 mins), macrophages (hours to days), fibroblasts (1 week) and myofibroblasts (2 weeks). Cytokines and growth factors secreted by macrophages include fibroblast growth factor (FGF) and transforming growth factors α and β (TGFα and TGFβ). Fibroblast growth factor is responsible for fibroblastic proliferation, and TGFβ1 for conversion of fibroblasts into myofibroblasts. After thermal injury, the use of topical curcumin, a non-competitive, selective PhK inhibitor that blocks PhK activity upstream of NF-kB activation, was found to be associated with more rapid and improved skin healing, as well as less severe or absent scarring.
Keywords
tissue injury, thermal injury, hypertrophic scarring, fibroblast proliferation, myofibroblast conversion
Mechanisms of Scarring after Thermal Injury
Wound healing following significant burns and scalds almost always result in hypertrophic scarring. In general, all tissues after injury are acutely infiltrated by many cell types (
Sequence of Events in Wound Healing Following Injury
We found that one of the earliest cells activated by tissue injury is the Langerhans cell, the activation of which is detected within 5 mins of epidermal injury (
Role of Fibroblasts and Myofibroblasts in Hypertrophic Scarring
Activated fibroblasts infiltrate the wound about a week after injury. TGF-β, which is chemotactic to activated fibroblasts, also stimulates fibroblastic proliferation (
The transformation of fibroblasts into myofibroblasts, which usually occurs 2 weeks after injury, is probably the key event in the formation of hypertrophic scarring. Myofibroblasts express α-smooth muscle actin and possess contractile properties resembling smooth muscle, resulting in generation of the forces that lead to wound contraction and hypertrophic scarring (
Signaling Pathways Induced by Injury
The molecule induced by injury and central to the wound healing process appears to be the transcription activator, NF-kB, which is detected as early as 30 mins after injury (
Curcumin (PhK Inhibitor) use after Thermal Injury
Phosphorylase kinase is a dual specificity kinase capable of transferring high energy phosphate bonds to both serine/threonine and tyrosine specific substrates (28). Most protein kinases can only transfer high energy phosphate bonds to substrates of a single specificity i.e. either serine/threonine or tyrosine. Phosphorylase kinase is a unique enzyme in which the spatial arrangements of the specificity determinants can be manipulated so that PhK can transfer high energy phosphate bonds from ATP to substrates of different specificities, such as serine/threonine and tyrosine residues (
Curcumin, the active ingredient of the spice, turmeric, is a non-competitive selective PhK inhibitor (
The following cases illustrate examples of patients with burns and scalds treated with topical curcumin gel that resulted in rapid healing and minimal or absent scarring.
Patient 1:
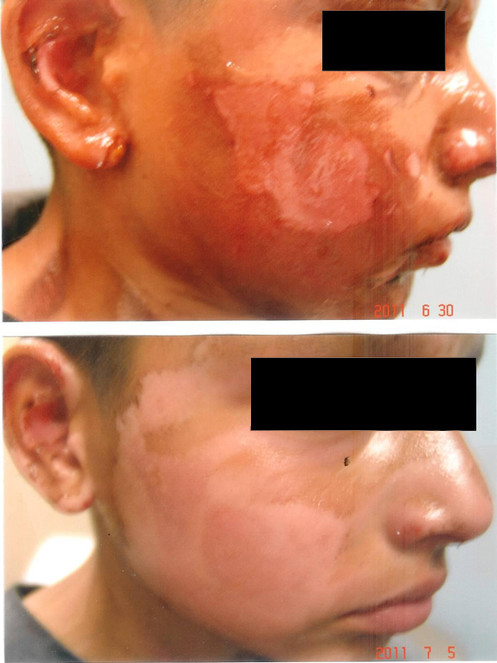
The patient is an 11 year old boy who sustained severe 2nd degree flash burns from pouring lighter fluid on warm barbeque coals. The heat from the ensuing fire singed his hair, eyelashes, forehead, ears, nose, cheeks and neck (Fig.
Burns from Barbeque Fire:
- (upper panel): Second degree burns from a barbeque fire before treatment with curcumin gel.
- (lower panel): Rapid healing 5 days after curcumin gel applied hourly.
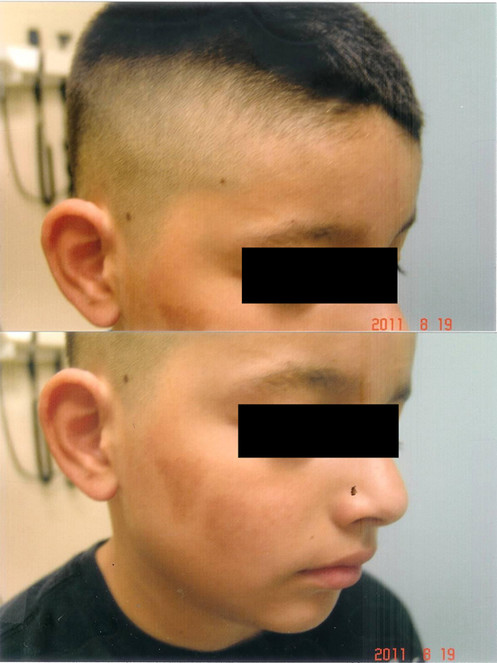
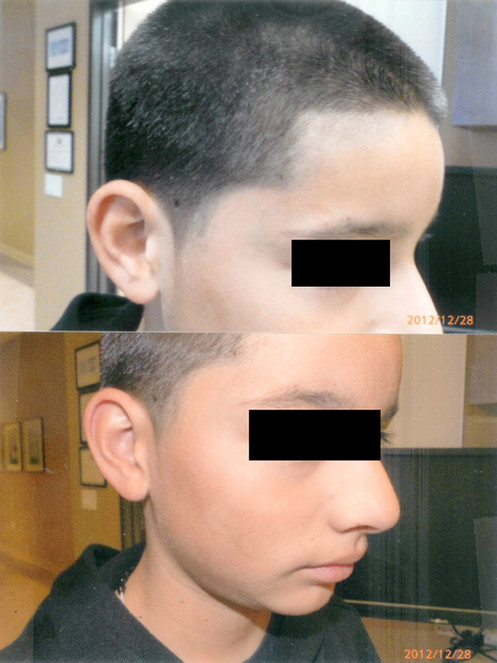
Burns from Barbeque Fire:
- (upper panel): The singed hair from the burn were removed by a hair-cut. At 6 weeks after curcumin gel, the skin of the forehead, eyelids and ears were completely healed, with some residual pigmentation over the forehead.
- (lower panel): Residual hyperpigmentation right cheek 6 weeks following curcumin gel treatment.
Patient 2:
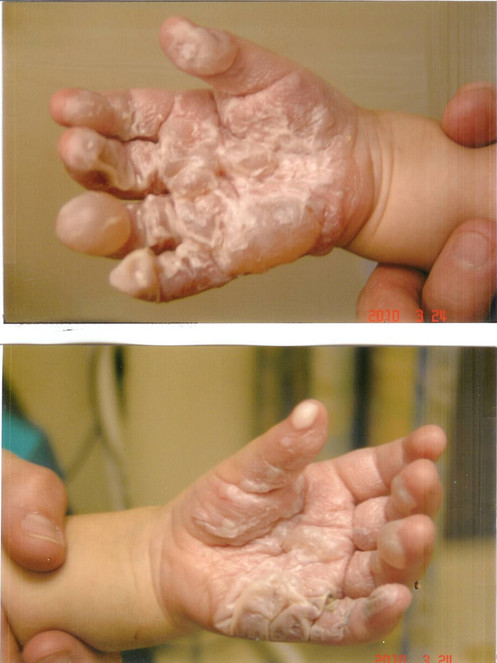
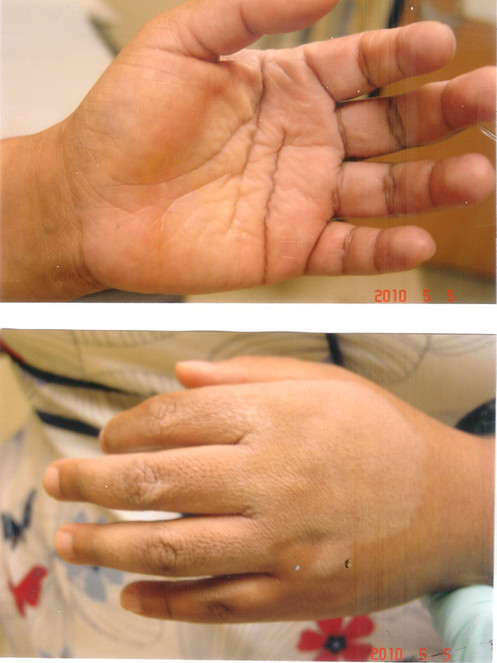
The patient was a 2-year old boy who sustained 2nd degree burns over the palms of both hands after falling into a camp-fire. He was seen at a number of emergency care centers, and treated with silvadene cream. When seen four days later, large blisters were seen over both palms (Fig.
Burns from a campfire see four days following injury, Note the presence of blisters suggesting at least second degree burns.
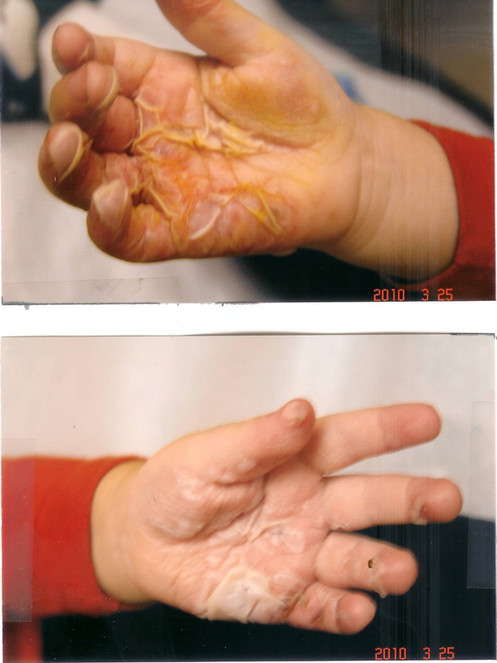
Improvement one day later after hourly application of curcumin gel. Pain and blistering were much improved.
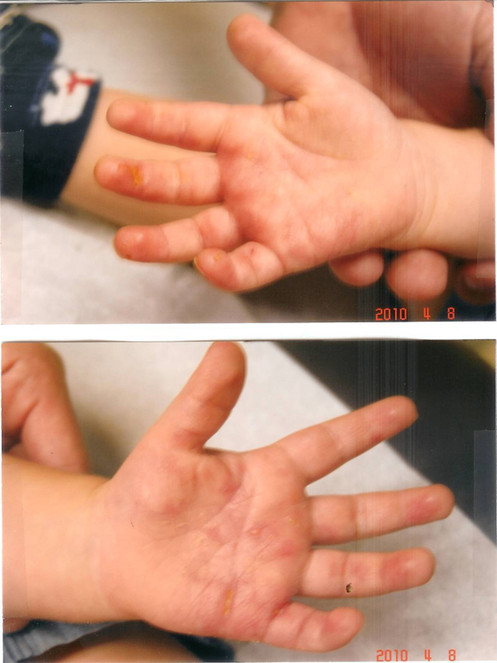
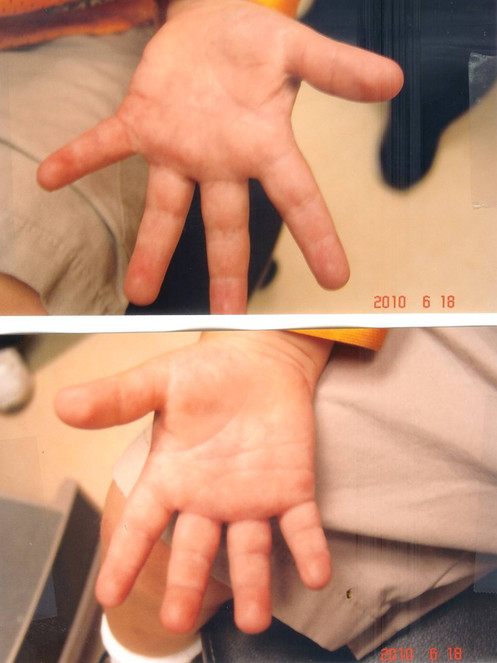
Significant healing observed with curcumin gel (multiple applications daily) was observed after two weeks. Note rapid re-epithelialization of most of the skin of both palms. Also note inability of the patient to fully extend his fingers.
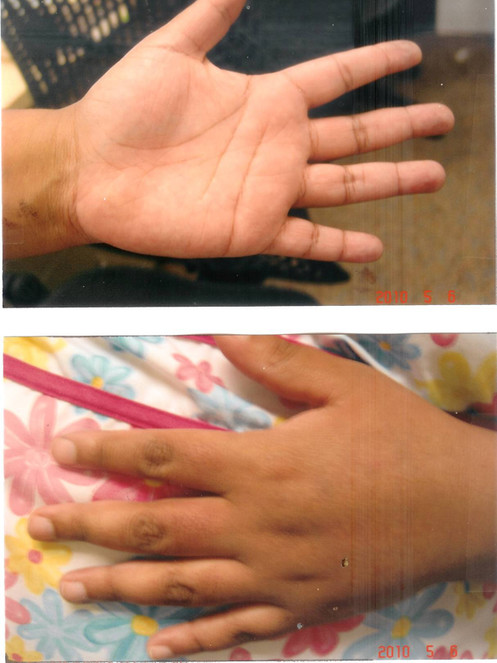
Patient 3:
The patient, a 35-year old female, sustained scalds to her left hand when she accidentally poured boiling water over the left hand. When seen one day later, early blister formation, suggestive of second degree injury, was observed both over the left palm and palmar aspect of all the fingers of her left hand (Fig.
Scald from boiling water seen one day after injury before curcumin gel. Note the presence of early blister formation associated with significant pain. Note also the inability of the patient to fully extend her fingers.
Potential Benefits of Curcumin in Thermal Injury - A Clinical Perspective
Wounds in adults, unlike fetal wounds, often heal with scarring (
In this review, we report the clinical outcome of several patients with burns and scalds treated with curcumin gel that resulted in minimal scarring or scar-free healing. We have previously reported similar beneficial outcomes with use of curcumin gel in post-surgical scars (
We noted that application of curcumin gel after burns and scalds is usually followed by rapid decrease in erythema, blistering, swelling and pain. Improvement in these clinical symptoms and findings strongly suggests that cytokine activity, in particular TNFα, is reduced in curcumin-treated wounds. TNFα is an inflammatory cytokine produced by many activated cells, and especially inflammatory cells such as T lymphocytes and macrophages. TNFα levels have been shown to be significantly increased in burns and other types of injury. By blocking PhK activity (
Conflicts of interest
Dr. Heng has shares in Omnicure Inc., a company that manufactures and markets topical curcumin gel.
References
-
Immunohistochemical Expression of TGF-β1 in Keloids and Hypertrophic Scars.The American Journal of Dermatopathology33(1):84‑91. https://doi.org/10.1097/dad.0b013e3181d0c3ad
-
Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations.Journal of Pediatric Surgery20(4):315‑319. https://doi.org/10.1016/s0022-3468(85)80210-4
-
Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl.Carcinogenesis23(1):143‑150. https://doi.org/10.1093/carcin/23.1.143
-
The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis.Journal of plastic, reconstructive & aesthetic surgery: JPRAS62(1):77‑84. https://doi.org/10.1016/j.bjps.2007.10.052
-
Ontogeny of the Skin and the Transition from Scar-Free to Scarring Phenotype during Wound Healing in the Pouch Young of a Marsupial, Monodelphis domestica.Developmental Biology169(1):242‑260. https://doi.org/10.1006/dbio.1995.1141
-
Traumatic spinal cord injury induces nuclear factor-kappaB activation.The Journal of neuroscience : the official journal of the Society for Neuroscience18(9):3251‑60.
-
Platelet isoforms of platelet-derived growth factor stimulate fibroblasts to contract collagen matrices.Journal of Clinical Investigation84(3):1036‑1040. https://doi.org/10.1172/jci114227
-
The Molecular and Cellular Biology of Wound Repair.Plenum Press, New York,391-423pp.
-
Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts.The Journal of Cell Biology122(1):103‑111. https://doi.org/10.1083/jcb.122.1.103
-
Scarring Occurs at a Critical Depth of Skin Injury: Precise Measurement in a Graduated Dermal Scratch in Human Volunteers.Plastic and Reconstructive Surgery119(6):1722‑1732. https://doi.org/10.1097/01.prs.0000258829.07399.f0
-
Mechanical Analysis of Hypertrophic Scar Tissue: Structural Basis for Apparent Increased Rigidity.Journal of Investigative Dermatology84(1):9‑13. https://doi.org/10.1111/1523-1747.ep12274528
-
Phenotypic and functional features of myofibroblasts in sheep fetal wounds.Differentiation56(3):173‑181. https://doi.org/10.1046/j.1432-0436.1994.5630173.x
-
Scar-free healing: from embryonic mechanisms to adult therapeutic intervention.Philosophical Transactions of the Royal Society B: Biological Sciences359(1445):839‑850. https://doi.org/10.1098/rstb.2004.1475
-
Super-thin abdominal skin pedicle flap for the reconstruction of hypertrophic and contracted dorsal hand burn scars.Burns34(3):400‑405. https://doi.org/10.1016/j.burns.2007.03.025
-
Fibroblasts, myofibroblasts, and wound contraction.The Journal of Cell Biology124(4):401‑404. https://doi.org/10.1083/jcb.124.4.401
-
Double-stranded RNA and bacterial lipopolysaccharide enhance sensitivit to TNF-α -mediated cell death.International Immunology2(9):903‑908. https://doi.org/10.1093/intimm/2.9.903
-
Changes in type of collagen during the development of human post-burn hypertrophic scars.Clinica Chimica Acta93(1):119‑125. https://doi.org/10.1016/0009-8981(79)90252-3
-
Curcumin targeted signaling pathways: basis for anti-photoaging and anti-carcinogenic therapy.International Journal of Dermatology49(6):608‑622. https://doi.org/10.1111/j.1365-4632.2010.04468.x
-
Signaling pathways targeted by curcumin in acute and chronic injury: burns and photo-damaged skin.International journal of dermatology52(5):531‑43. https://doi.org/10.1111/j.1365-4632.2012.05703.x
-
Early events after skin injury: Observations on changes in Langerhans cells and Birbeck granules with role of phosphorylase kinase in the injury pathway.International Journal of Clinical and Experimental Physiology1(4):283. https://doi.org/10.4103/2348-8093.149759
-
Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters.British Journal of Dermatology143(5):937‑949. https://doi.org/10.1046/j.1365-2133.2000.03767.x
-
Wound healing in adult skin: aiming for perfect regeneration.International Journal of Dermatology50(9):1058‑1066. https://doi.org/10.1111/j.1365-4632.2011.04940.x
-
Phosphorylation Meets Ubiquitination: The Control of NF-κB Activity.Annual Review of Immunology18(1):621‑663. https://doi.org/10.1146/annurev.immunol.18.1.621
-
Burn Scar Biomechanics after Pressure Garment Therapy.Plastic and Reconstructive Surgery136(3):572‑581. https://doi.org/10.1097/prs.0000000000001507
-
Reconstruction of the Burned Hand.Plastic and Reconstructive Surgery127(2):752‑759. https://doi.org/10.1097/prs.0b013e3181fed7c1
-
Expression of Transforming Growth Factor Beta 1, 2, and 3 Proteins in Keloids.Annals of Plastic Surgery43(2):179‑184. https://doi.org/10.1097/00000637-199902000-00013
-
The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum.The American journal of pathology78(1):71‑100.
-
Wound Healing--Aiming for Perfect Skin Regeneration.Science276(5309):75‑81. https://doi.org/10.1126/science.276.5309.75
-
Rapid induction and clearance of TGFβ1 is an early response to wounding in the mouse embryo.Developmental Genetics14(3):225‑238. https://doi.org/10.1002/dvg.1020140309
-
Early excision and skin grafting versus delayed skin grafting in deep hand burns (a randomised clinical controlled trial).Burns37(1):36‑41. https://doi.org/10.1016/j.burns.2010.02.005
-
Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing.Proceedings of the National Academy of Sciences85(13):4894‑4897. https://doi.org/10.1073/pnas.85.13.4894
-
NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase.Nature401(6748):82‑5. https://doi.org/10.1038/43466
-
Phosphorylation of Serine 68 in the I B Kinase (IKK)-binding Domain of NEMO Interferes with the Structure of the IKK Complex and Tumor Necrosis Factor - induced NF - B Activity.Journal of Biological Chemistry283(1):76‑86. https://doi.org/10.1074/jbc.m708856200
-
Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase.FEBS Letters341(1):19‑22. https://doi.org/10.1016/0014-5793(94)80232-7
-
Prevention and treatment of postburn scars and contracture.World Journal of Surgery16(1):87‑96. https://doi.org/10.1007/bf02067119
-
Activation of Transcription Factor NF- B Is Suppressed by Curcumin (Diferuloylmethane).Journal of Biological Chemistry270(42):24995‑25000. https://doi.org/10.1074/jbc.270.42.24995
-
A comparative study of spray keratinocytes and autologous meshed split-thickness skin graft in the treatment of acute burn injuries.Wounds: a compendium of clinical research and practice27(2):31‑40.
-
Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm.Cell76(2):301‑314. https://doi.org/10.1016/0092-8674(94)90337-9
-
Identification of a p65 Peptide That Selectively Inhibits NF- B Activation Induced by Various Inflammatory Stimuli and Its Role in Down-regulation of NF- B-mediated Gene Expression and Up-regulation of Apoptosis.Journal of Biological Chemistry279(15):15096‑15104. https://doi.org/10.1074/jbc.m311192200
-
Polarized Th2 Cytokine Production in Patients with Hypertrophic Scar Following Thermal Injury.Journal of Interferon & Cytokine Research26(3):179‑189. https://doi.org/10.1089/jir.2006.26.179
-
Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation.Genes & Development9(22):2723‑2735. https://doi.org/10.1101/gad.9.22.2723
-
Curcumin induces apoptosis through the mitochondria-mediated apoptotic pathway in HT-29 cells.Journal of Zhejiang University SCIENCE B10(2):93‑102. https://doi.org/10.1631/jzus.b0820238
-
Deep dermal fibroblasts contribute to hypertrophic scarring.Laboratory Investigation88(12):1278‑1290. https://doi.org/10.1038/labinvest.2008.101
-
Immunohistochemical localization of growth factors in fetal wound healing.Developmental Biology147(1):207‑215. https://doi.org/10.1016/s0012-1606(05)80018-1
-
The Zinc Finger Mutation C417R of I- B Kinase Impairs Lipopolysaccharide- and TNF-Mediated NF- B Activation through Inhibiting Phosphorylation of the I- B Kinase Activation Loop.The Journal of Immunology172(4):2446‑2452. https://doi.org/10.4049/jimmunol.172.4.2446
-
Phosphorylase kinase, a metal ion-dependent dual specificity kinase.The Journal of biological chemistry268(24):17683‑6.