|
BioDiscovery : Research Article
|
|
Corresponding author: Dragomira S. Stoyanova (dragomira.stoyanova@abv.bg)
Academic editor: Lidia Sakelarieva
Received: 11 Oct 2017 | Accepted: 09 Dec 2017 | Published: 15 Dec 2017
© 2017 Dragomira Stoyanova, Iliana Ivanova, Orlin Angelov, Todorka Vladkova
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Stoyanova D, Ivanova I, Angelov O, Vladkova T (2017) Antibacterial activity of thin films TiO2 doped with Ag and Cu on Gracilicutes and Firmicutes bacteria. BioDiscovery 20: e21596. https://doi.org/10.3897/biodiscovery.20.e21596
|
|
Abstract
This article aims to explore the antibacterial activity of thin films of TiO2 doped with Ag and Cu using two types of Gram-negative and Gram-positive test bacteria with clinical significance (Gracilicutes and Firmcutes bacteria). The thin films (thickness of about 60 nm) were deposited on glass substrates by radio frequency magnetron co-sputtering (r.f. power of 50 W) of TiO2 target with Ag and Cu pieces on its surface in an Ar atmosphere (0.8 Pa) without heating during the deposition. The total surface area of the Ag was 60 mm2 and that of the Cu was 100 mm2. Bacillus cereus, Staphylococcus epidermidis, Salmonella enterica, Escherichia coli and Pseudomonas sp. were used as test strains. The antibacterial actvity of the films was evaluated by the classical Koch's method and optical density measurements. The bactericidal effect was established at different time points between 30 min and 90 min for Pseudomonas sp. and S. enterica. The Firmicutes bacteria B. cereus and S. epidermidis were killed at the 4th and 8th hour of the treatment, respectively. The effect on E. coli was bacteriostatic until the 10th hour. The results were confirmed by assessment of the bacterial dehydrogenase activity. The studied thin films of TiO2 co-doped with Ag and Cu have a potential for application as antibacterial coatings.
Keywords
TiO2 thin films, bactericidal effect, Gram-positive and Gram-negative bacteria, clinically significant strains
Introduction
The antibacterial effect of the nanomaterials is due to different processes based on their surface biochemical functionality, partial dissolution of their components and mechanical antibacterial effect of their surface morphology. The utilisation of nanostructured materials as antimicrobial agents depends on their high surface to volume ratio. TiO2 is a widely used material for scientific studies and different applications in clinical practice due to its biocompatibility and high stability at chemical treatments and environmental conditions. The modification of the TiO2 band gap through doping with different chemical elements does not affect its ultraviolet light activity and increases the photocatalytic activity in sunlight. It is known that transition metals (W, V, Ag, Cu etc.) or non-metals (S, C, N2) doped TiO2 has a lower band gap than pure TiO2 and can be used to increase the production of reactive oxygen species (ROS) under visible light irradiation in solutions containing undesired pollutants
TiO2 porous coatings with different Ag concentration (0, 0.95, 1.36 and 1.93 wt%, respectively) prepared through combining magnetron sputtering with micro-arc oxidation were presented by
According to
To summarise, no detailed research has been reported for antimicrobial activity of TiO2 thin films doped with Ag and Cu and deposited through r.f.magnetron co-sputtering. This is the reason to study the antibacterial effect of these films and compare their influence on Gracilicutes and Firmicutes bacteria.
Material and methods
Preparation of thin film coated glass substrates
The thin films were deposited on glass substrates by r.f. magnetron co-sputtering (13.56 MHz; r.f. power of 50 W; in Ar atmosphere at 0.8 Pa) of the TiO2 target with Ag and Cu pieces on the surface in its maximum erosion zone without heating during the deposition. The total surface area of Ag (4 similar pieces) was 60 mm2 and that for Cu (4 similar pieces) was 100 mm2. The size of the glass substrates was 20 x 25 mm, preliminarily treated by a solution of H2SO4 : H2O2 (1:1) to ensure the adhesion of coating. The thickness of the films was about 60 nm, measured by a Taylor Hobson profilometer. The optical properties of the films were analysed through transmittance and reflectance measurements by a Shimadzu 3600 spectrophotometer.
Bacterial strains
The following bacteria were used in this study: E. coli ATCC 10536, Pseudomonas putida ATCC 12633, Bacillus cereus ATCC 7050, Staphylococcus epidermidis ATCC 12228 and Salmonella enterica serotype choleraesius DSMC 4224; all supplied by the Bulgarian National Bank for Industrial Microorganisms and Cell Cultures (NBIMCC).
Antimicrobial test
Diffusion assay
A diffusion assay was used for fast screening. The test bacterial strains were grown in the most suitable growth medium for each species at 180 rpm for 18 h. The microbial density of 0.5 - 0.8 was determined according to McFarland. The aliquots of 100 μl microbial suspension were randomly spread on a solid nutrient medium (Conda, Spain or ISO 10712 for Pseudomonas) and the glass matrices of the investigated material were added. The plates were left for 20 h at 4 - 6oC to afford diffusion of the nanoparticles and after, theywere cultivated for 24 h at 25oC for P. putida and 37oC for all other bacteria. The sterile zones formed around the samples were measured in mm (+ 0.5).
Disinfection time
Determination of the disinfection time was conducted for 24 h in a light-dark regime with illumination for 15 min during the sampling in Corning® Costar® TC-Treated six-well plates. Two wells were used for the control as bare glasses (without thin films) and the other four wells with thin film coated glass substrates were placed and sterilised by 30 min UV-irradiation. In the well with the blank control, a sterile nutrient liquid medium was added over the thin sterile film without bacteria to measure the thin film dissolution and light absorption of the liquid (Nutrient broth, Conda, Spain or ISO 10712 for Pseudomonas putida). In the remaining 5 wells, a liquid culture medium inoculated with a test microorganism with initial concentration (OD 0.001) of overnight bacterial culture in the exponential phase was added. The inoculum and medium quantity in all variants were the same for all samples and controls at the beginning of the experiment. The optical density was measured every hour with a Zeiss spectrophotometer at λ = 610 nm. Also, the determination of surviving bacteria was studied through the classical Koch's method. Every 2 h, consecutive decimal dilutions were prepared for determination of the bacterial amount of the samples and the controls. After plating of 100 μl bacterial suspension on a solid nutrient medium without thin films and cultivation at 25oC for P. putida and 37oC for all other bacteria, counting of the bacterial colonies was conducted.
The maximum growth rate (μ) of the control and the samples was calculated through the equation:
μ= (lgx-lgx0)/(lg 2)(t-t0) = (lg N-lg N0)/0,3(t-t0) (1),
where:
x0, N0 - the initial quantity of the bacteria, evaluated by classical Koch's method
x, N - the final quantity of the bacteria, evaluated by classical Koch's method
t - t0 - incubation time.
Assessment of the dehydrogenase activity
Iodonitro-tetrazolium chloride (INT) was used for assessment of the dehydrogenase activity inhibition. It is known that INT has a higher sensitivity to dehydrogenase enzymes than triphenyl tetrazolium chloride (TTC), but TTC is more resistant to Cu ions in the suspensions. This was the reason to use both reagents INT and TTC for its parallel determination. No differences were detected between them. The optical density of the stained supernatant was measured at λ = 465-480 nm. The control is usually stained as bright-red and the samples as yellow or light red colour depending on the inhibition of enzyme activity. The optical density results were recalculated using a standard value for the amount of colourant obtained and the amount of protein determined as described by
SEM observations
A Scanning Electron Microscope, JOEL model JSM 5510 (Japan) was used for observation of the bacterial surface morphology on the thin films.
Atomic Flame Absorption Spectrometry
Atomic Flame Absorption Spectrometry (Perkin Elmer Aanalyst 400) was used for determination of Ag and Cu in the liquid and control medium as described by
CMe=(Ko-S)/A.CFU.mL-1 (2),
where:
Ko - dissolved NPs and ions from control (thin film in nutrient medium without bacteria),
S - dissolved NPs and ions from sample (thin film in nutrient medium with bacteria),
A - atomic mass of the studied metal,
CFU.mL-1 - Colony Forming Units (CFU) initial bacterial quantity in one ml of culture medium.
The values of the dissolved and permanently associated with the organic molecules Ag nanoparticles and ions were accounted. The results were represented as mol/cell.
Results
Inhibitory effect in static conditions
The result from the diffusion assay test have shown that the inhibition zone was different for the test bacteria: 11 mm for E. coli and S. epidermidis, 12 mm for B. cereus and 13-14 mm for Pseudomonas sp. It can be concluded that E. coli and S. epidermidis are the most resistant to the tested films.
Disinfection time
The disinfection time of the studied thin films was determined for all tested bacteria during cultivation in the liquid medium.
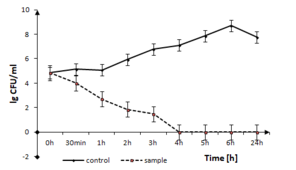
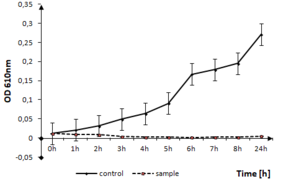
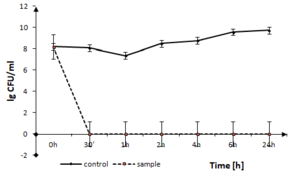
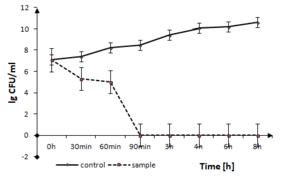
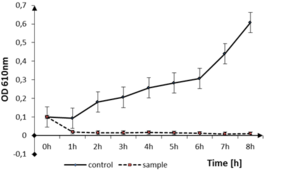
The disinfection time of the coating to Gram-positive pathogen, B. cereus is presented in Fig.
B. cereus growth in the presence of TiO2:Ag:Cu thin films. The solid line represents the bacterial quantity for the control without TiO2:Ag:Cu film and dashed line represents the quantity of surviving cells in sample with TiO2:Ag:Cu film, both in logarithmic scale.
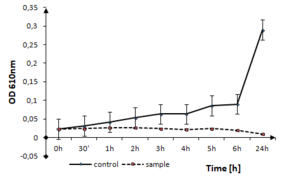
b: optical density measurements
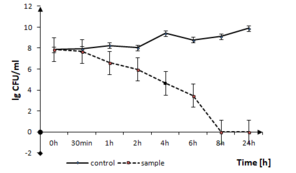
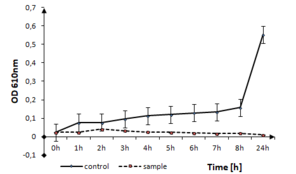
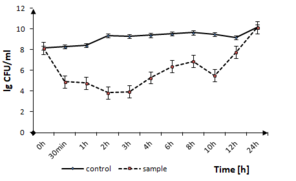
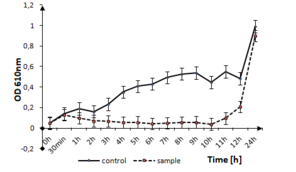
The effect of tested thin films on the other Firmicutes bacteria, like S. epidermidis can be seen in Fig.
S. epidermidis growth in the presence of TiO2:Ag:Cu thin films. The solid line represents the bacterial quantity for the control without TiO2: Ag: Cu film and the dashed line represents the quantity of surviving cells in the sample with TiO2:Ag:Cu film.
b: Optical density measurements
Fig.
P. putida growth in the presence of TiO2:Ag:Cu thin films. The solid line represents the bacterial quantity for the control without TiO2:Ag:Cu film and the dashed line represents the quantity of surviving cells in the sample with TiO2:Ag:Cu film, both in logarithmic scale.
b: optical density measurements
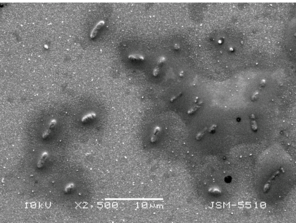
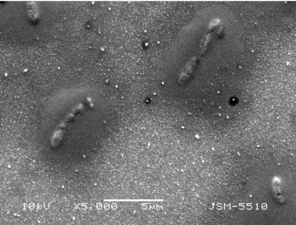
The image of P. putida cells on thin TiO2:Ag:Cu film by Scanning Electron Microscope with different magnifications.
b: x5000
The influence of nanostructured thin films TiO2:Ag:Cu on the growth of S. enterica is represented in Fig.
S. enterica growth in the presence of TiO2:Ag:Cu thin films. The solid line represents the bacterial quantity for the control without TiO2:Ag:Cu film and the dashed line represents the quantity of surviving cells in the sample with TiO2:Ag:Cu film in logarithmic scale.
b: optical density measurements
E. coli growth in the presence of TiO2:Ag:Cu thin films. The solid line represents the bacterial quantity for the control without TiO2:Ag:Cu film and the dashed line represents the quantity of surviving cells in the sample with TiO2:Ag:Cu film in logarithmic scale.
b: optical density measurements
Atomic Flame Absorption Spectrometry
The bactericidal concentration of Ag and Cu (nanoparticles and ions) in solution was measured for the representatives of two types of the tested bacteria - Firmicutes and Gracilicutes. The values for blank control (organic nutrient medium with a thin film without bacteria) were determined as 5.2 mg/l for Ag and as 4.2 mg/l for Cu. For the samples with bacteria (B. cereus) and thin films, these values were 4.8 mg/l for Ag in organic growth medium and 3.6 mg/l for Cu also in organic medium; the difference between the control and the sample is the amount engulfed by the Bacillus cells (105 CFU/ml) - 0.6 mg/l Cu and 0.4 mg/l Ag.
In the synthetic culture medium ISO 10712, the content of dissolved Ag was 1.04 mg/land Cu was 2.4 mg/l in the sample with 108 CFU P. putida per ml.
Discussion
In this study, five bacterial strains were exposed to TiO2:Ag:Cu thin films. The results indicated that the tested materials have a bactericidal effect on the bacterial cells in different time periods.
The bactericidal effects of TiO2:Ag:Cu was evident after 30 min and 90 min of exposure of P. putida and S. enterica determined by the Koch's method. Only on E. coli, known as a highly adaptable bacterial species, the effect was bacteriostatic. However, a strong retention until the 10th hour of the treatment was observed. For the tested Firmicutes bacteria, the bactericidal effect was observed at the 4th and 8th hours for B. cereus and S. epidermidis respectively. This effect is within the time limits which do not allow biofilm formation. As reported by
At the end of the experiment, only 2.8% retention in the maximum growth rate at the 24th hour for E. coli was observed. In contrast, all the other bacterial strains were completely deactivated. The maximum growth rate counted on the controls data obtained by Koch's method (test bacterial strains without nanomaterials) were 0.404, 0.279, 0.214, 1.472 and 0.283 for B. cereus, S. epidermidis, P. putida and S. enterica respectively.
The toxic effect was confirmed by an assessment of the bacterial dehydrogenase activity. As is well known, this activity is proof of normal cell metabolism and its inhibition is due to destruction of the bacterial cell wall and membrane. The stress response in the treated E. coli cells was the dehydrogenase acivity increase in the sample in comparison to the control. The other bacterial strains, in contact with the E. coli, have shown no enzyme activity when tested with INT and TTC. Only E. coli retains the dehydrogenase activity at the 24th hour; the formazan quantity in the sample measured was OD470 = 0.804 compared to the OD470 = 0.671 in the control and the calculated enzyme activity in the sample was 0.253 FNU (Formazin Nephelometric Units), compared to the control (0.08 FNU). The bacteriostatic effect and the adaptation of E. coli to the tested nanomaterials could be explained by the ability of this bacterial species to activate special transport pumps for export of the metal ions out of the cell. This fact is the mechanism of stress response as has been reported by
The tested material demonstrated bactericidal concentration on P. putida at 3.77 x 10-7 mol/cell for Cu and 9.64 x 10-11 mol/cell for Ag. For the Firmicutes representative B. cereus, the toxic effect was observed at 3.71 x 10-8 mol/cell for Ag and 9.44 x 10-6 mol/cell for Cu. These results confirmed that the Gracilicutes bacteria are more sensitive to dissolved Ag and Cu from the studied thin films.
The results from the atomic absorption spectrometry proved a partial dissolution of metal atoms and ions from the tested thin films. Probably as a result of the faster penetration of Ag and Cu atoms in the cell, they cause a quicker toxic effect on the Gracilicutes bacteria. Moreover, due to the established low concentration of Ag and Cu in our experiment, low cytotoxicity to human cells can also be expected. A concentration of 5 μg/ml Ag nanoparticles (NPs) affects the epithelial cell line, according to
The neurotoxicity of Ag NPs with an average size of 15 nm at a concentration of 10 μg/ml for 24 h was investigated using the dopaminergic neuronal cell line PC12 by
The effects of the tested thin films depend on the structure of the bacterial cell wall
The study of
Conclusions
The antibacterial activity of TiO2 thin films doped with Ag and Cu using two types of Gram-negative and Gram-positive test bacteria with clinical significance (Gracilicutes and Firmicutes bacteria) were tested. The antibacterial activity of the thin films was evaluated by the classical Koch's method and optical density and dehydrogenase activity measurements. The bactericidal effect was established at different time periods between 30 min - 90 min for Pseudomonas sp. and S. enterica. The Firmicutes bacteria B. cereus and S. epidermidis were killed at the 4th and 8th hours of the treatment respectively. The effect against E. coli was bacteriostatic until the 10th hour. The studied TiO2:Ag:Cu thin films have potential for application as antibacterial agents on clinically significant bacterial strains. For the future perspective, other technological variants with similar combinations will be tested.
Acknowledgements
Sofia University Scientific Fund (project 80-10-12/12.04.2017) and Bulgarian National Scientific Fund (grand DCOST 01/14/08.12.2016) are gratefully acknowledged for the financial support of this investigation performed in the frame of COST Action TD 1305 "Improved Protection of Medical Devices Against Infections".
References
-
The use of nanoparticles to control oral biofilm formation.Journal of Dental Research89:1175‑1185. https://doi.org/10.1177/0022034510377794
-
Antibacterial Properties of Silver Doped TiO2 Nanoparticles Synthesized via Sol-Gel Technique.Macromolecular Researchhttps://doi.org/10.1007/s13233-016-4066-9
-
Interference of Silver, Gold, and Iron Oxide Nanoparticles on Epidermal Growth Factor Signal Transduction in Epithelial Cells.ACS Nano5(12):10000‑10008. https://doi.org/10.1021/nn203785a
-
Determination of Silver by Flame Atomic Absorption Spectrometry after Preconcentration on Naphthalene Modified with Dithizone.Journal of the Chinese chemical society56:725‑728. https://doi.org/10.1002/jccs.200900108
-
Antimicrobial activity of titania/silver and titania/copper films prepared by CVD.Journal of Photochemistry and Photobiology A: Chemistry216(2-3):283‑289. https://doi.org/10.1016/j.jphotochem.2010.09.017
-
TiO2 Photocatalysis and related surface phenomena.Surface Science Reports63:515‑582. https://doi.org/10.1016/j.surfrep.2008.10.001
-
Prokaryotic tests for assessment of metal toxicity in soil and water.Ecological engineering and environment protection95‑103.
-
An extended spectrum bactericidal titanium dioxide (TiO2) coating for metallic implants: in vitro effectiveness against MRSA and mechanical properties.Journal of Materials Science: Materials in Medicine22(2):381‑387. https://doi.org/10.1007/s10856-010-4204-4
-
UV-enhanced bioactivity and cell response of micro-arc oxidized titania coatings.Acta Biomaterialia4:1518‑1529. https://doi.org/10.1016/j.actbio.2008.03.005
-
A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization.Journal of Materials Science: Materials in Medicine16(10):883‑888. https://doi.org/10.1007/s10856-005-4422-3
-
Review of Antibacterial Activity of Titanium-Based Implants’ Surfaces Fabricated by Micro-Arc Oxidation.Coatings7https://doi.org/10.3390/coatings7030045
-
The photocatalytic properties of amorphous TiO2 composite films deposited by magnetron sputtering.Research on Chemical Intermediates38(2):487‑498. https://doi.org/10.1007/s11164-011-0365-0
-
Antibacterial and Biocompatible Titanium-Copper Oxide Coating May Be a Potential Strategy to Reduce Periprosthetic Infection: An In Vitro Study.Clinical Orthopaedics and Related Research®475(3):722‑732. https://doi.org/10.1007/s11999-016-4713-7
-
Silver Nanoparticles Supported on TiO2 and Their Antibacterial Properties: Effect of Surface Confinement and Nonexistence of Plasmon Resonance.Materials Sciences and Applications5:895‑903. https://doi.org/10.4236/msa.2014.512091
-
Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli.Annals of Microbiology60:75‑80.
-
Comparison of HIPIMS sputtered Ag- and Cu-surfaces leading to accelerated bacterial inactivation in the dark.Surface & Coatings Technology250:14‑20. https://doi.org/10.1016/j.surfcoat.2014.02.029
-
Antibiofilm activity of epoxy/Ag-TiO2 polymer nanocomposite coatings against Staphylococcus aureus and Escherichia coli.Coatings5(5):95‑114. https://doi.org/10.3390/coatings5020095
-
Antimicrobial applications of nanotechnology: methods and literature.International Journal of Nanomedicine7:2767‑2781. https://doi.org/10.2147/IJN.S24805
-
Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria.Journal of Colloid and Interface Science275(1):177‑182. https://doi.org/10.1016/j.jcis.2004.02.012
-
Antimicrobial activity of silver nitrate against periodontal pathogens.J period res36:108‑113. https://doi.org/10.1034/j.1600-0765.2001.360207.x/full
-
Measurement of cytoplasmic copper, silver, and gold with a lux biosensor shows copper and silver, but not gold, efflux by the CopA ATPase of Escherichia coli.FEBS Letters546(391):394. https://doi.org/10.1016/S0014-5793(03)00640-9
-
Antibacterial Activity of Glutathione-Coated Silver Nanoparticles against Gram Positive and Gram Negative Bacteria.Langmuir28:8140‑8148. https://doi.org/10.1021/la3003838
-
Effect of silver nanoparticles on Pseudomonas putida biofilms at different stages of maturity.Journal of Hazardous Materials290:127‑133. https://doi.org/10.1016/j.jhazmat.2015.02.073
-
Toxicity effects of four typical nanomaterials on the growth of Escherichia coli, Bacillus subtilis and Agrobacterium tumefaciens.Environmental Earth Sciences65:1643‑1649. https://doi.org/10.1007/s12665-011-1139-0
-
Expression changes of dopaminergic system-related genes in PC12 cells induced by manganese, silver, or copper nanoparticles.NeuroToxicology30(6):926‑933. https://doi.org/10.1016/j.neuro.2009.09.005
-
Antibacterial property of Ag nanoparticle-impregnated N-doped titania films under visible light.Scientific Reports5(1): . https://doi.org/10.1038/srep11978
-
Antibacterial property, angiogenic and osteogenic activity of Cu-incorporated TiO2 coating.Journal of Materials Chemistry B2(39):6738‑6748. https://doi.org/10.1039/c4tb00923a
-
Titanium dioxide (TiO2) nanoparticles induce bacterial stress response detectable by specific lux biosensors.Nanotechnologies in Russia6:401‑406. https://doi.org/10.1134/s1995078011030165
-
The dual function of Cu-doped TiO2 coatings on titanium for application in percutaneous implants.Journal of Materials Chemistry B4(21):3788‑3800. https://doi.org/10.1039/c6tb00563b
-
Microstructure and cytotoxicity evaluation of duplex-treated silver-containing antibacterial TiO2 coatings.Materials Science and Engineering C, Materials for Biological Applications45:402‑410. https://doi.org/10.1016/j.msec.2014.07.002
-
Synthesis of bioactive ceramic on the titanium substrate by micro-arc oxidation.Journal of Biomedical Materials Research Part A90(90):438‑445. https://doi.org/10.1002/jbm.a.31902
-
Biological activity and antibacterial property of nano-structured TiO2 coating incorporated with Cu prepared by micro-arc oxidation.Journal of Materials Science and Technology29:237‑244. https://doi.org/10.1016/j.jmst.2012.12.015