Introduction
Breast cancer has high incidence and is a common cause of cancer-related death in women worldwide. The most common site of distant metastasis in breast cancer patients is the skeleton [
Osteonecrosis of the jaw is characterized by bone death as a consequence of a wide variety of systemic and local factors that compromise bone blood flow [
Bisphosphonate-associated osteonecrosis of the jaw (BONJ) is a concerning side effect of bisphosphonates. Bisphosphonates play an important role in the therapy of breast cancer patients with osseous metastases. Depending on the ligands, bisphosphonates are separated into nitrogen-containing and non-nitrogen-containing bisphosphonates. The former inhibit the mevalonate pathway, and the latter are integrated into the ATP molecule. Both mechanisms result in a cytotoxic effect on the osteoclasts. There is further evidence that bisphosphonates reduce and delay skeletal-related events and reduce pain and thereby improve quality of life [
In 2003, after alert initial observations by Marx [
According to the American Association of Oral and Maxillofacial Surgeons [
The literature suggests BRONJ incidence rate of 0.028% to 4.3% [
The appearance of bisphosphonate-associated osteonecrosis is identical to the appearance of osteoradionecrosis in patients who develop it after undergoing head and neck irradiation [
Similar to osteoradionecrosis, the radiographic features of BRONJ have many similarities to that of chronic osteomyelitis [
Treatment of BRONJ is difficult and depending on the stage of the disease. Therapy ranges from simple mouth rinses for asymptomatic exposed bone to debridement and huge resections of the maxilla or the mandible, the latter having a large impact on the quality of life for these patients [
It is important to realize that BRONJ is a new clinical entity, and new cases are being reported daily. We report two cases of patients with osteonecrosis of the jaw associated with bisphosphonate therapy. We discuss management options, as well as recent guidelines for treatment.
Case 1
A 61 year-old woman was referred to our clinic in November 2009 to evaluate “an infection at the left premolar-molar area of the mandible and left canine area of the maxilla after a dental extraction that, after four months, had not responded to treatment with antibiotics”. In discussing her medical history the patient pointed out that she had been diagnosed with breast cancer in 2006 and had been treated with chemotherapy. She had been receiving bisphosphonate therapy for four years, and then she was receiving zoledronic acid at the time of the extractions. She also had a history of vertebrate fractures, and zoledronic acid intravenous administration in doses of 4 mg during the previous 3 years.
The patient reported the presence of non-healing extraction sites after undergoing the extraction of an
unrestorable tooth, no. 11, by her general dentist five months before her initial visit to us. In addition, teeth nos. 19 and 20 had extreme mobility because of periodontal problems and were extracted. Her general dentist carried out these procedures without flap reflection or primary closure. Three months after the extraction of the teeth, her dentist observed that the patient had delayed healing of all three extraction sites and exhibited exposed necrotic bone in the extraction sites in spite of antibiotic therapy. The patient was re-examined a month later, with no improvement in her condition. In addition, she reported that she had not disclosed her bisphosphonate use to either her general dentist or previous oral surgeon until after the extractions had been performed as she did not think this medication was relevant to her current dental condition or treatment.
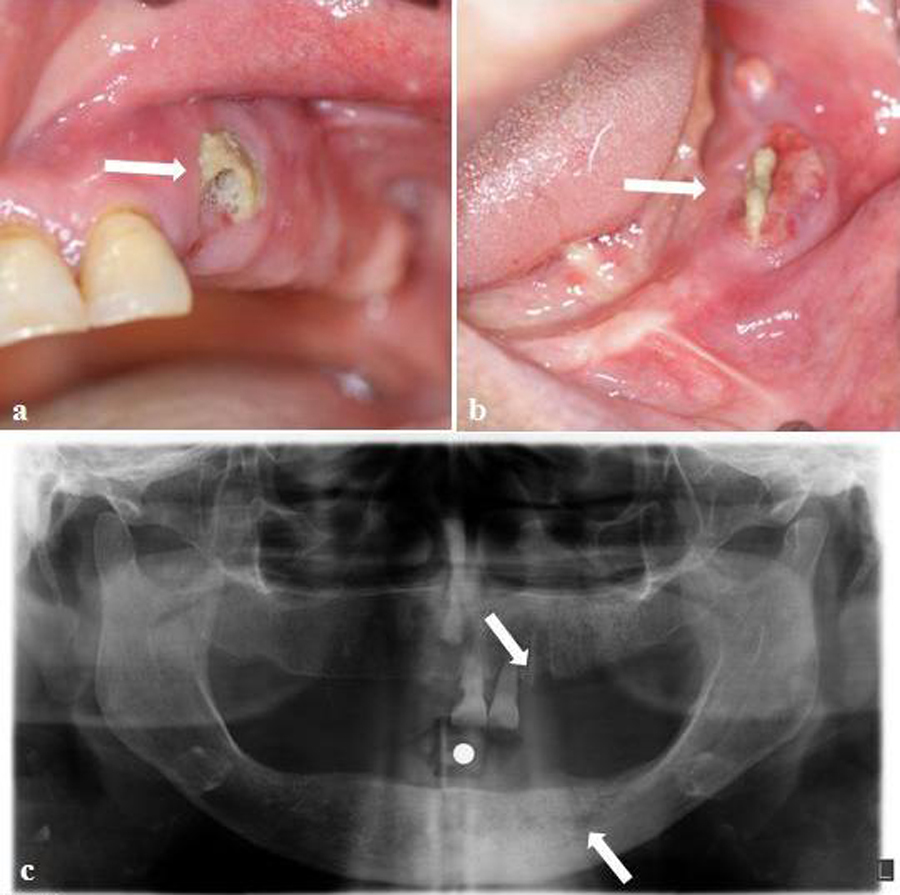
In intraoral exploration non-healing extraction sockets were found and the surrounding alveolar bone was partially exposed. The associated mucosa appeared red and inflamed. Purulent exudate (Figure 1 a, b) and an extraoral fistula were also present (Figure 1 c). BRONJ was diagnosed by a maxillofacial surgeon based on the following criteria: exposed bone in the maxilla or mandible associated with pain and soft-tissue swelling, unhealed necrotic bone after dental work, long-term use of bisphosphonates, not having received radiation therapy, and poorly demarcated radio-opaque area of the affected bone on X-ray. However, under a local anesthetic the minimum debridement of a necrotic bone was performed; a biopsy was taken, and a microbiological culture was made. From the results, diagnosis of BRONJ was confirmed.
The differential diagnosis of the lesion included a fungal infection secondary to her immunocompromised status, as well as osteoradionecrosis. The oral surgeon ruled out the latter because the patient reported that she had not received radiation therapy.
After consulting with the patient’s oncologist, treatment with intravenous zoledronic acid was discontinued. After the discontinuation of bisphosphonate therapy, we instructed the patient to use a 0.12% chlorhexidine oral rinse three times a day and initiated therapy with an antibiotic (amoxicillin-clavulanic acid 1g twice daily) by oral administration and non-steroid anti-inflammatories. Ten days later, the patient revisited our department as requested. Under local anesthesia and antibiotic coverage, an oral surgeon explored the patient’s extraction sites and debrided, and excised the diseased and necrotic osseous, and soft tissues. He removed necrotic alveolar bone until reaching what appeared to be bleeding vital osseous tissue. He removed necrotic alveolar bone until reaching what appeared to be bleeding vital osseous tissue. He irrigated the site close to the operative site and instructed the patient to use a chlorhexidine oral rinse three times a day and initiated long-term antibiotic therapy with 500 mg of penicillin VK twice a day. At the three-week postoperative visit, the subject had no complaints. Healing in the affected area was progressing well and no bleeding or purulent exudate were present.
The subject failed to report for her five-month check-up. At the five-month postoperative visit (in May 2010),
the patient had evolved towards a clinical and radiographic improvement, and the pain had diminished almost completely although the soft tissue around the sockets had not healed. Purulent exudate was observed and a great bone area of sequestrum was delimited in the extraction areas (Figure 1d).
An oral surgeon performed the previous treatment procedures. He also extracted teeth nos. 9 and 10. He irrigated the site, undermined the flap margins to gain tension-free flap closure and closed the operative site with multiple interrupted polypropylene sutures. We instructed the patient to use a chlorhexidine oral rinse three times a day and initiated antibiotic therapy as previously explained.
The patient returned to our department two weeks later for a follow-up check. The oral examination revealed evidence of soft-tissue breakdown of the flap closure, with visible necrotic alveolar bone in the region of tooth no.11. Purulent exudate was observed. The oral surgeon debrided the site again, under local anaesthesia and antibiotic coverage, until he observed viable bleeding bone. He irrigated the site and closed the operative site. The oral surgeon instructed the patient to use a chlorhexidine oral rinse three times a day and initiated antibiotic treatment (amoxicillin-clavulanic acid 1g twice daily plus metronidazole 1 g daily). At the three-week postoperative visit, the subject had no complaints. Healing in the affected area was progressing well and no bleeding or purulent exudate were present.
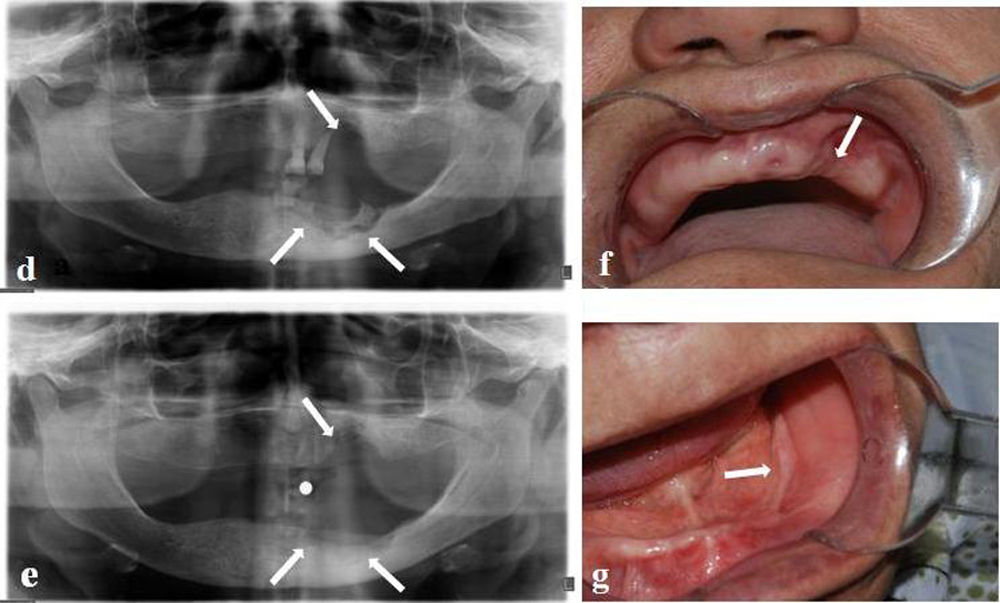
We followed up the patient every month to reevaluate the affected areas and to ensure that they had not become suppurative. The patient was treated with antibiotics until the areas healed. The outcome, although slow and torpid, has been a significant improvement, and at this moment, 11 months after the initial diagnosis, our patient is asymptomatic and has no evidence of infection. There was a complete mucosal coverage. In the panoramic X-ray, the image has been standardized (Figure 1 e) and we can see that the intraoral soft tissue has completely healed (Figure 1 f, g). The patient is being followed up in scheduled check-ups.
Case 2
A 49-year-old female patient was referred to the Department of Oral Diagnosis and Radiology at the Faculty of Dentistry with chief complaints of pain in the left mandible. The patient reported the presence of non-healing extraction sites after undergoing the extraction of unrestorable teeth nos. 18 and 19 by her dentist two years prior her initial visit to us. Her dentist carried out the procedures without flap reflection or primary closure. Four months after the extraction of the teeth her dentist noted that the patient had a delayed healing of the extraction sites and exhibited exposed necrotic bone in them. The patient was re-examined five weeks later and no improvement was observed in her condition in spite of antibiotic therapy.
In discussing her medical history she pointed out that she had been diagnosed with metastatic breast cancer (MBC) and had been treated with the intravenous (IV) bisphosphonate drug zoledronate prescribed by her oncologist. She had been taking 4 milligrams monthly for
the treatment of her MBC. At that point she had been receiving bisphosphonate therapy for five years since being diagnosed with breast cancer. In addition to MBC, which was diagnosed two years before she came to our department, the patient also had, according to her medical history, hypertension, asthma and diabetes. She was on zoledronic acid at the time of the dental extractions. In addition, she reported that she had not disclosed her bisphosphonate use to either her general dentist or previous oral surgeon until after the extractions had been performed as she did not think this medication was relevant to her current dental condition or treatment.
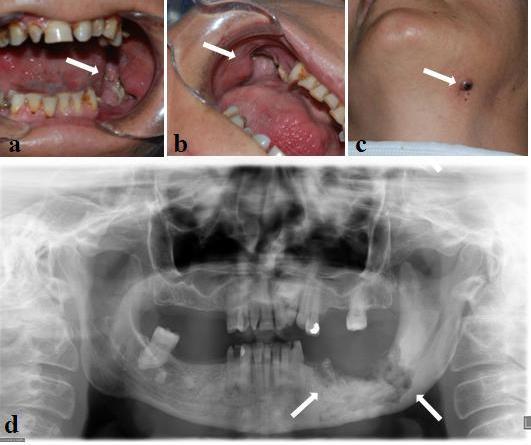
In oral examination, there was a significant bony depression and the surrounding alveolar bone was partially exposed (Figure 2 a, b). The associated mucosa appeared red and inflamed. Purulent exudate was also present. The left mandible had a large area of osteonecrosis that became secondarily infected, leading to the formation of an extraoral fistula (Figure 2 c). A panoramic radiograph taken at the subject’s visit showed a deep bony defect in the left premolar- molar area (Figure 2 d).
BRONJ was diagnosed by a maxillofacial surgeon based on the same criteria as in case 1. We recommended that she undergo treatment consisting of antibiotics and surgical debridement. The oral surgeon irrigated the site. Although we considered removing bisphosphonate therapy from the patient’s chemotherapy regimen, the patient’s oncologist and other members of the medical team did not advise doing so owing to the profound beneficial effects that these drugs were having on the patient’s quality of life. We instructed the patient to use a 0.12% chlorhexidine oral rinse three times a day and initiated therapy with an antibiotic (amoxicillin-clavulanic acid 1g twice daily plus metronidazole 1 g daily) by oral administration and non-steroid anti-inflammatories. In the following three weeks, the pain diminished almost completely. The oral surgeon recommended the patient to have surgical intervention to resect the left mandibular body from the angle to the canine region and to insert a titanium reconstruction bar. However, the patient refused any surgical intervention but continued to be followed up. Unfortunately, shortly after her last visit to our department, the patient died of metastatic breast carcinoma.
Discussion
Bisphosphonates are important drugs that are used widely for several medical purposes including the prevention and treatment of bone metastases associated with cancer [
Although causality may never be proven, emerging experimental and epidemiologic studies have established a strong association between bisphosphonate treatment and the appearance of painful exposed nonvital bone in the jaws after oral surgery has been reported in the last decade [
Bisphosphonates (BPs) are divided into two types depending on administration method - intravenous infusion and oral administration. Patients taking oral BPs manifested less bone exposure and milder symptoms compared to patients on IV BPs [
Recently, cases of BRONJ in patients with various types of cancer receiving intravenous bisphosphonates, such as pamidronate and zoledronic acid to control and treat metastatic bone disease, have been reported in the medical and dental literature of [
Usually patients with BRONJ are asymptomatic in the initial stage but with time severe symptoms appear causing extensive bone sequestrums, suppuration, and mucosa or cutaneous fistula with continuous and intense pain [
When tissues are severely infected, patients may complain of acute pain and lack of sensory sensation (paresthesia). This may be an indication of peripheral nerve compression [
Radiographic findings vary from no evidence of bone changes to bone sclerosis, lytic areas and obvious sequestration [
In the early stages of BRONJ no significant changes may appear on radiographs. Later, radiographic changes may mimic classic periapical inflammatory lesions or osteomyelitis. Other radiographic findings include non-healing extraction site, widening of the periodontal ligament space and osteosclerotic lamina dura [
Procedures for the treatment strategies of patients using these drugs have been outlined by task forces from the American Dental Association [
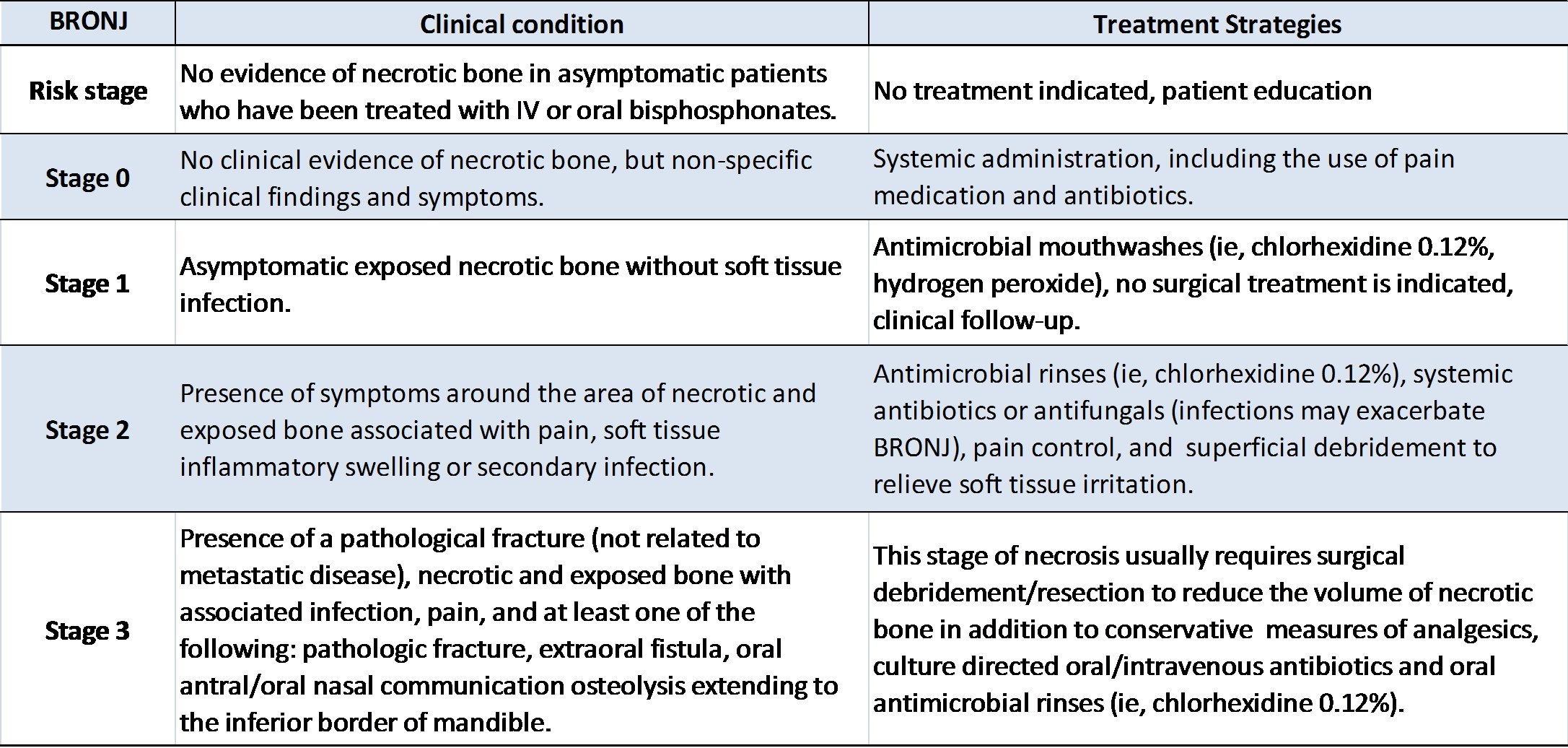
The staging system and treatment strategies have been proposed by these institutions (Table 1). In our first case, the patient was in stage 2 and this treatment plan (local debridement, sequestrectomy, use of antibiotics, daily chlorhexidine mouth rinsing) was performed successfully. Several different adjunctive treatments (ie, hyperbaric oxygen [
BRONJ is a recently documented oral complication, and proven effective therapeutic measures have not yet been identified. The management of patients receiving oral or IV bisphosphonate therapy is mainly preventive in nature [
They should also be given information about BRONJ and be made aware of the early symptoms of development of this condition [
Management of dental care for patients receiving bisphosphonate therapy should be directed at reducing the future need for dentoalveolar surgery [
There has been much discussion as to the benefits of stopping the drug for a period of time, a so called ‘drug holiday’. It is suggested that cessation of BP treatment allows for regeneration of osteoclasts and therefore improved bone turnover, and this has some support from studies looking at biochemical markers of bone turnover, but there is no consensus on the duration of drug holiday necessary for this to occur. Any decision on temporary cessation of BP therapy must obviously be taken in conjunction with the prescribing physician and whether this is possible will be determined by the clinical indication for BP therapy [
Shannon et al. stated that it is uncertain whether stopping bisphosphonate therapy will help manage BRONJ once the drug has been incorporated into bone. Because the half-lives of most bisphosphonates are months to years, there is no strong evidence that
discontinuation of treatment before dental procedures will significantly affect the occurrence of BRONJ, but some suggest that, if the person has already undergone oral bisphosphonate therapy for longer than three years, or for less than three years while taking corticosteroids, the bisphosphonate treatment should be stopped at least three months before a surgical procedure and, if possible, not resumed until osseous healing occurs because the combination of the two drugs may increase the risk of BRONJ. Particular care should be taken with people taking IV bisphosphonates, who should be advised to avoid elective invasive dental procedure during therapy; if the person develops BRONJ, dental surgery may exacerbate the condition. Careful consultation between the dentist, physician, and patient is necessary before any modification or cessation of treatment is considered [
Although interrupting or reducing bisphosphonate exposure might have beneficial effects on the development of BRONJ, it is unlikely that bisphosphonate discontinuation for a short period of time will allow significant reduction in the risk of developing BRONJ. As a result, it is not clear how long bisphosphonate interruption or reduction is required to achieve significant decrease in the risk of development of BRONJ [
Some authorities reported that preventative dental management reduced the risk of BRONJ among patients with malignancy treated with IV bisphosphonates. These results proposed that, althoughthe risk of BRONJ is not eliminated, dental evaluations and treatment prior to initiating IV bisphosphonate therapy among cancer patients decreases the risk of the condition [
In conclusion, bisphosphonates are the drugs of choice for many life-threatening diseases. It is important that health professionals, especially dentists, oncologists and oral surgeons be aware of the association between bisphosphonate treatment and delayed wound healing and osteonecrosis of the jaws. They should perform a comprehensive oral examination in patients before they begin any chemotherapy regimen. They should also follow existing guidelines for dental consultation for the prevention of oral complications of cancer therapy. Patients should be educated by specialists about the possible dental side effects of bisphosphonate therapy and take the necessary preventive measures to keep potential side effects to a minimum.
In addition, continued high-quality clinical research on the prevention, risk reduction, and treatment of BRONJ needs to be developed further so that more precise decisions regarding risk, prognosis, proper dental management option, and results can be established for patients with BRONJ.
References
- Pavlakis N, Schmidt R, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev 2005; 20: CD003474.
- Ficarra G, Beninati F. Bisphosphonate-related osteonecrosis of the jaws: an update on clinical, pathological and management aspects. Head Neck Pathol 2007; 1: 132-140.
Reference Link - Berenson JR, Hillner BE, Kyle RA, Anderson K, Lipton A, Yee GC, et al. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. J Clin Oncol 2002; 20: 3719-3736
Reference Link - Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, et al. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol 1998; 16: 593-602
- Bittner T, Lorbeer N, Reuther T, Böhm H, Kübler AC, Müller-Richter UD. Hemimandibulectomy after bisphosphonate treatment for complex regional pain syndrome: A case report and review on the prevention and treatment of bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113:41-7. Epub 2011 Mar 31.
- Mariotti A. Bisphosphonates and osteonecrosis of the jaws. J Dent Educ;72:919-929.
- Tarassoff P, Csermak K. Avascular necrosis of the jaws: risk factors in metastatic cancer patients. J Oral Maxillofac Surg 2003; 61: 1238-1239.
Reference Link - Walter C, Al-Nawas B, du Bois A, Buch L, Harter P, Grötz KA. Incidence of bisphosphonate-associated osteonecrosis of the jaws in breast cancer patients. Cancer 2009; 115:1631-1637.
Reference Link - Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003; 61: 1115-1117.
Reference Link - Rosenberg TJ, Ruggiero S. Osteonecrosis of the jaws associated with the use of bisphosphonates. J Oral Maxillofac Surg 2003; 61: 60 (letter)
Reference Link - Migliorati CA. Bisphosphanates and oral cavity avascular bone necrosis. J Clin Oncol 2003; 15: 4253-4254.
Reference Link - Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 2005; 63: 1567-1575.
Reference Link - Bagan JV, Jimenez Y, Murillo J, Hernandez S, Poveda R, Sanchis JM, et al. (). Jaw osteonecrosis associated with bisphosphonates: multiple exposed areas and its relationship to teeth extractions. Study of 20 cases. Oral Oncol 2006; 42: 327-329.
Reference Link - Dimitrakopoulos I, Magopoulos C, Karakasis D. Bisphosphonateinduced avascular osteonecrosis of the jaws: a clinical report of 11 cases. Int J Oral Maxillofac Surg 2006; 35: 588-593.
Reference Link - Migliorati CA, Siegel MA, Elting LS. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol 2006; 7: 508-514.
Reference Link - American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. Approved by the Board of Trustees, September 25, J Oral Maxillofac Surg 2007; 65: 369?76.">Reference Link
- Walter C, Grotz KA, Kunkel M, Al-Nawas B. Prevalence of bisphosphonate associated osteonecrosis of the jaw within the field of osteonecrosis. Support Care Cancer 2007; 15: 197-202.
Reference Link - Solomon DH, Mercer E, Woo SB, Avorn J, Schneeweiss S, Treister N. Defining the epidemiology of bisphosphonate-associated osteonecrosis of the jaw: prior work and current challenges. Osteoporos Int 2012;Jun 16. [Epub ahead of print]
Reference Link - Wang EP, Kaban LB, Strewler GJ, Raje N, Troulis MJ. Incidence of osteonecrosis of the jaw in patients with multiple myeloma and breast or prostate cancer on intravenous bisphosphonate therapy. J Oral Maxillofac Surg 2007; 65: 1328-1331.
Reference Link - Aguiar Bujanda D, Bohn Sarmiento U, Cabrera Suarez MA, Aguiar Morales J. Assessment of renal toxicity and osteonecrosis of the jaws in patients receiving zoledronic acid for bone metastasis. Ann Oncol 2007; 18: 556-560.
Reference Link - Sanna G, Preda L, Bruschini R, Cossu Rocca M, Ferretti S, Adamoli L, et al. Bisphosphonates and jaw osteonecrosis in patients with advanced breast cancer. Ann Oncol 2006; 17: 1512-1516.
Reference Link - Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: review of 63 cases. J Oral Maxillofac Surgery 2004; 62: 527?534.
- Torres SR, Chen CS, Leroux BG, Lee PP, Hollender LG, Santos EC, et al. Drew SP, Hung KC, Schubert MM. Mandibular cortical bone evaluation on cone beam computed tomography images of patients with bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol 2012 113(5):695-703. Epub 2012 Apr 12.
- Infante Coss?o P, Cabezas Maci?n A, P?rez Ceballos JL, Palomino Nicas J, Guti?rrez P?rez JL. Bisphosphonate-related osteonecrosis of the jaw in patients with multiple myeloma. Med Oral Patol Oral Cir Bucal 2008;1:E52-7.
- Lipton A, Theriault RL, Hortobagyi GN, Simeone J, Knight RD, Mellars K, Reitsma DJ, et al. Pamidronate prevents skeletalcomplications and is effective palliative treatment in women with breastcarcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer 2000; 88: 1082-1090.
Reference Link - Walter C, Al-Nawas B, Frickhofen N, Gamm H, Beck J, Reinsch L,et al. Prevalence of bisphosphonate associated osteonecrosis of the jaws in multiple myeloma patients. Head Face Med 2010; 8: 6:11.
- Aparicio A, Gardner A, Tu Y, Savage A, Berenson J, Lichtenstein A. In vitro cytoreductive effects on multiple myeloma cells induced by bisphosphonates. Leukemia 1998; 12: 220-229.
Reference Link - Gralow J. Evolving role of bisphosphonates in women undergoing treatment for localized and advanced breast cancer. Clin Breast Cancer 2005; 5: 54-62.
Reference Link - Kumar A, Loughran T, Alsina M, Durie BG, Djulbegovic B. Management of multiple myeloma: a systematic review and critical appraisal of published studies. Lancet Oncol 2003; 4: 293-304.
Reference Link - Lee MV, Fong EM, Singer FR, Guenette RS. Bisphosphonate treatment inhibits the growth of prostate cancer cells. Cancer Res 2001; 61:2602-2608.
- Yoneda T, Hashimoto N, Hiraga T. Bisphosphonate actions on cancer. Calcif Tissue Int 2003; 73: 315-318.
Reference Link - Santini D, Fratto ME, Vincenzi B, La Cesa A, Dianzani C, Tonini G. Bisphosphonate effects in cancer and inflammatory diseases: in vitro and in vivo modulation of cytokine activities. BioDrugs 2004; 18: 269-278.
Reference Link - Kumar V, Pass B, Guttenberg SA, Ludlow J, Emery RW, Tyndall DA, Padilla RJ. Bisphosphonate-related osteonecrosis of the jaws: a report of three cases demonstrating variability in outcomes and morbidity. J Am Dent Assoc 2007; 138: 602-609.
- Grotz KA, Walter C, Kuttner C, Al-Nawas B. Relevance of bisphosphonate long-term therapy in radiation therapy of endosteal jaw metastases. Strahlenther Onkol 2007; 183: 190-194.
- Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws ? 2009 update. J Oral Maxillofac Surg 2009; 67: 2-12.
Reference Link - Sarasquete ME, Garcia-Sanz R, Marin L, Alcoceba M, Chillon MC, Balanzategui A, et al. Bisphosphonate-related osteonecrosis of the jaw is associated with polymorphisms of the cytochrome P450 CYP2C8 in multiple myeloma: a genome-wide single nucleotide polymorphism analysis. Blood 2008; 112: 2709.
Reference Link - Zuffetti F, Bianchi F, Volpi R, Trisi P, Del Fabbro M, Capelli M, et al. Clinical application of bisphosphonates in implant dentistry: histomorphometric evaluation. Int J Periodontics Restorative Dent 2009; 29: 31-39.
- Durie B, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med 2005; 353:99-102.
Reference Link - Migliorati CA, Casiglia J, Epstein J, Jacobsen PL, Siegel MA, Woo SB. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc 136(12):1658-1668. Review. Erratum in: J Am Dent Assoc 2006; 137: 26.
- Mehrotra B, Ruggiero S. Bisphosphonate complications including osteonecrosis of the jaw. Hematology Am Soc Hematol Educ Program 2006; 515: 356-360. Review.
Reference Link - Hellstein JW, Marek CL. Bisphosphonate osteochemonecrosis (bis-phossy jaw): is this phossy jaw of the 21st century? J Oral Maxillofac Surg 2005; 63: 682-689.
Reference Link - Ruggiero S, Gralow J, Marx RE, Hoff AO, Schubert MM, Huryn JM, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Practice 2006; 2: 7-14.
Reference Link - Thorn JJ, Hansen HS, Specht L, Bastholt L. Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J Oral Maxillofac Surg 2000; 58: 1088-1093.
Reference Link - Chiandussi S, Biasotto M, Dore F, Cavalli F, Cova MA, Di Lenarda R. Clinical and diagnostic imaging of bisphosphonate-associated osteonecrosis of the jaws. Dentomaxillofac Radiol 2006; 35: 236-243.
Reference Link - Edwards BJ, Hellstein JW, Jacobsen PL, Kaltman S, Mariotti A, Migliorati CA. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc 2006; 137:1144-1150.
- Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007; 22:1479-1491.
Reference Link - Bocanegra-P?rezS, Vincente-BarreroM, Sosa-Hern?ndezM, Knezevic M, Castellano-NavarroJM, Rodr?guez-MillaresJ. Bisphosphonate- associated osteonecrosis of the jaw.Aproposal for conservative treatment. Med Oral Pathol Oral Cir Bucal 2008; 13: 770-773.
- Freiberger JJ. Utility of hyperbaric oxygen in treatment of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 2009; 67(5 Suppl): 96-106.
Reference Link - Harper RP, Fung E. Resolution of bisphosphonate-associated osteonecrosis ofthemandible: possible application for intermittent low dose parathyroid hormone [rhPTH(1-34)]. J Oral Maxillofac Surg 2007; 65: 573-580.
Reference Link - Curi MM, Cossolin GSI, Koga DH, Ara?jo SR, Feher O, dos Santos MO, et al. Treatment of a vascular osteonecrosis of the mandible in cancer patients with a history of bisphosphonate therapy by combining bone resection and autologous platelet-rich plasma: report of 3 cases. J Oral Maxillofac Surg 2007; 65: 349-355.
- Vescovi P, Merigo E, Meleti M, Fornaini C, Nammour S, Manfredi M. Nd:YAG laser biostimulation of bisphosphonate-associated necrosis of the jaw bone with and without surgical treatment. Br J OralMaxillofac Surg 2007; 45: 628-632.
Reference Link - Stubinger S, Dissmann JP, Pinho NC, Saldamli B, Seitz O, Sader R. A preliminary report about treatment of bisphosphonate related osteonecrosis of the jaw with Er:YAG laser ablation. Lasers Surg Med 2009; 41: 26?30.
- Agrillo A, Ungari C, Filiaci F, Priore P, Iannetti G. Ozonetherapyin the treatment of a vascular bisphosphonate-related jaw osteonecrosis. J Craniofac Surg 2007; 18: 1071-1075.
Reference Link - Merigo E, Manfredi M, Meleti M, Guidotti R, Ripasarti A, Zanzucchi E, D'Aleo P, et al. Bone necrosis of the jaws associated with bisphosphonate treatment: a report of twenty-nine cases. Acta Biomed 2006; 77: 109-117.
- Lacy MQ, Dispenzieri A, Gertz MA, Greipp PR, Gollbach KL, Hayman SR et al. Mayo Clinic Consensus Statement for the use of bisphosphonates in Multiple Myeloma. Mayo Clin Proc 2006; 81: 1047-1053.
Reference Link - McLeod NM, Brennan PA, Ruggiero SL. Bisphosphonate osteonecrosis of the jaw: a historical and contemporary review. Surgeon. 2012; 10: 36-42. Epub 2011 Oct 7.
Reference Link - Shannon J, Shannon J, Modelevsky S, Grippo AA. Bisphosphonates and osteonecrosis of the jaw. J Am Geriatr Soc. 2011; 59: 2350-2355
Reference Link - Z Jabbour, M El-Hakim, P Mesbah-Ardakani, JE Henderson, R Albuquerque Junior. The outcomes of conservative and surgical treatment of stage 2 bisphosphonate-related osteonecrosis of the jaws: a case series, Int. J. Oral Maxillofac. Surg. 2012; Jun 13 [Epub ahead of print]
Reference Link - Ripamonti CI, Maniezzo M, Campa T, Fagnoni E, Brunelli C, Saibene G, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009; 20: 137-145.
Reference Link