Introduction
Among the peptide family, short peptides are very appealing for drug discovery and development because of their cost-effectiveness, possibility of oral
administration, and simplicity to perform molecular structural and quantitative structure-activity studies [
Various RGD-containing peptides have been increasingly developed for adapting to versatile applications including tumor imaging and therapy, drug delivery
vector, targeted gene transfer, and biomaterial or tissue engineering. Significant progress has been made in the discovery and development of integrin α vα3-specific linear and cyclic RGD peptide analogs such as cilengitide and c(RGDfK) for cancer therapy, as well as targeted delivery
of cancer imaging and therapeutic agents. Examples of such radio-imaging agents include [99mTc]apticide, which can be used in imaging deep vein thrombosis
[
Despite the few RGD analogues being approved for clinical use, development of orally active RGD peptidomimetics have been significantly hindered because of
low bioavailabilities. This is largely due to the metabolic lability of this class of compounds in the presence of proteases and peptidases and because of
their high polarity and charge. Wang et al. [
In this context, drug design based on the RGD structure may provide opportunity for targeted drug deliveries, high resolution imaging of cancerous tissues and organs, as well as new chemotherapeutic treatments for cancer.
Following our long-term program for the design of biologically active peptides based on the non-protein amino acids, we synthesized several short
RGD-mimetics containing the sequence Xaa-GD, where Xaa is Arg or Arg-mimetic (Figure 1) with a view to improve its
cytotoxic activity
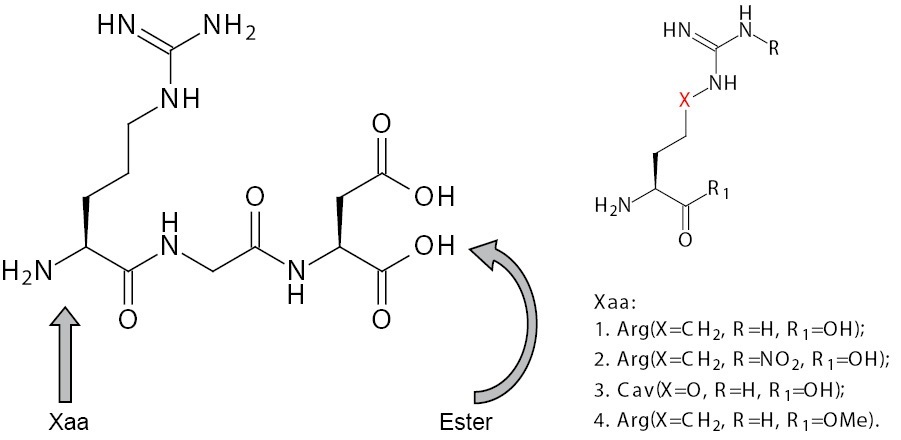
The purpose of this study, therefore was to investigate the cytotoxic activities of RGD and its newly synthesized mimetics (2-4) on non-tumour 3T3 cells and tumour cell lines HepG2 and MCF-7.
Results
The RGD analogues were synthesized following a procedure previously reported [
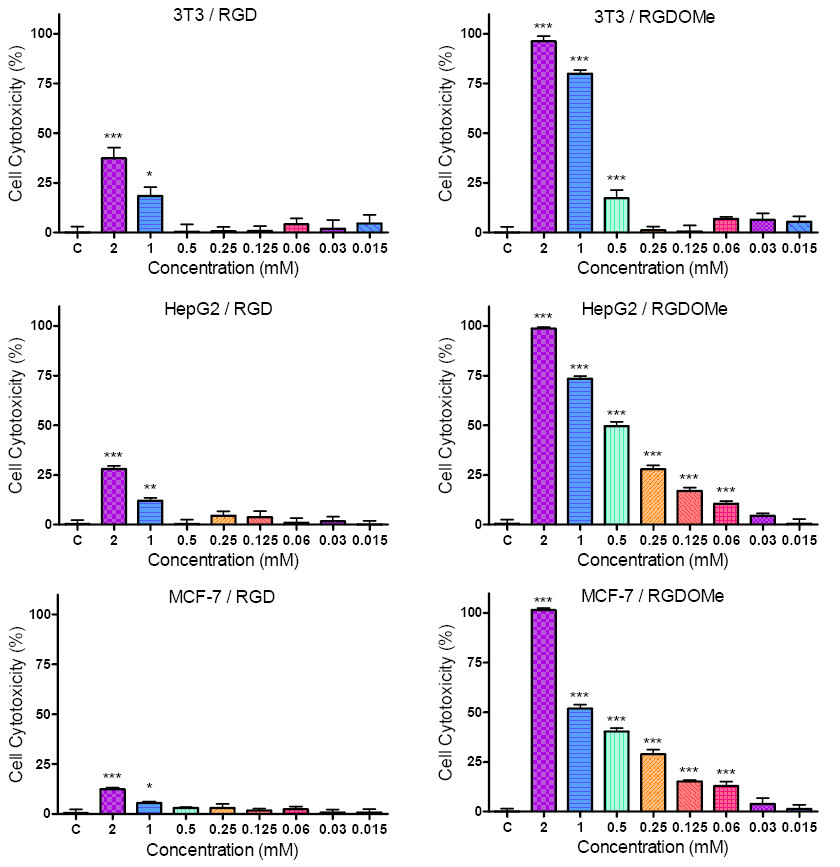
The results of the cytotoxicity of RGD and RGD-OMe on non-tumour 3T3 cells and tumour cell lines HepG2 and MCF-7 are shown in Figure 3. The cells were exposed for 24 hours to different concentrations (ranging from 2 to 0.015 mM) of the compounds. As seen in Figure 3. treatment of the cell lines resulted in a dose-dependent reduction of the number of the viable cells. The RGD analogue RGD-OMe exhibited higher cell growth inhibitory effects on the three cell lines compared to its parent compound. Statistically reliable results for the cytotoxic action of RGD-OMe on HepG2 and MCF-7 cells were achieved for the most of concentrations used.
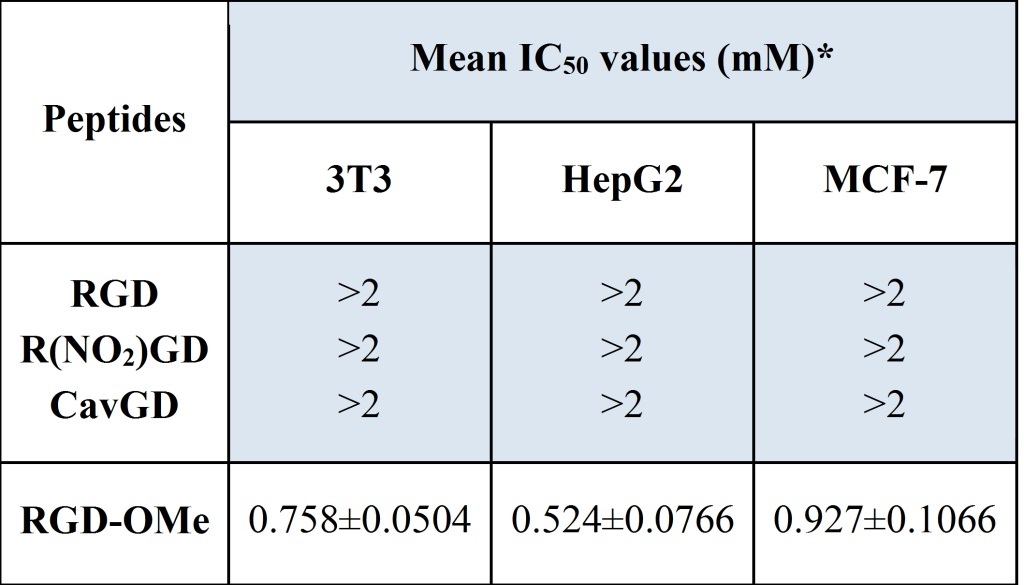
The cytotoxic activity of the synthesized peptides (1-4) is demonstrated by calculating the half maximal inhibitory concentrations (IC50 values), shown in Table 1. IC50 values of 1-3, in 3T3, HepG2 and MCF-7 are >2 mM (Table 1). IC50 values of RGD-OMe in 3T3, HepG2 and MCF-7 are 0.758, 0.524 and 0.927 respectively. Evidently HepG2 is the most sensitive of the three cell lines to the cytotoxic effects of the RGD-OMe.
HepG2 cells, treated with RGD and RGD-OMe were investigated for damages in their genomic DNA by neutral variant of Comet assay. The obtained by us Comet assay data confirmed that the RGD and RGD-OMe revealed certain DNA damaging activity, i.e. genotoxicity, which was increasing with the modifications of the compound (results not published). In an attempt to look deeper in the details of the observed genotoxicity we searched for consequence of the compounds on the cell cycle. The FACS analysis, however, has not proposed evidence of distinctive influence of the studied compounds on the stages of cell cycle. This result show that even strongly pronounced the genotoxic activity of the compounds is not connected with certain check point of the cell cycle (results not published).
Discussion
Peptides containing unusual amino acids are used in a multitude of applications including structural activity studies, diagnostics, and new drug discovery, improvements in bioavailability, molecular markers, and biologically active pharmaceuticals.
We decided to carry out various structural modifications on N- and C-terminal of the RGD molecule [
As shown by several authors, Cav a structural analogue of Arg, possesses growth retardation activity toward tumor cells in culture and
experimental tumors in vivo [
The cytotoxic activities of peptides 2 and 3 on 3T3 and HepG2 cells were examined (results not shown) but only the highest concentration used (2 mM) revealed cell growth inhibitory effect. Next, we decided to perform an experiment with C-terminal modified RGD. For this purpose we synthesized RGD-OMe 4.
The results showed that the cell growth inhibitory effects of 4 are significantly higher than those of RGD on the three cell lines. Evidently, the modification in the carboxylic group of RGD with simple esterification increases the cell growth inhibitory effects of the parent compound.
The effect of RGD-OMe is higher in the case of the tumor cells HepG2 in comparison with non-tumour cells 3T3. This observation suggests that the modification in the carbocylic group is significant for the antitumour action of RGD.
According to our recent investigations, as well as our further work, which is in progress, these new members of RGD are easily transformed into derivatives with improved pharmacological properties, or they can be used as building blocks for combinatorial peptide libraries.
Materials and Methods
Synthesis
The peptides 1 and 2 were synthesized manually by the solid phase method using Fmoc chemistry on (2-Chloro)chlorotrityl resin (Iris Biotech GmbH, Germany) using Fmoc/tBu methodology. Fmoc groups were removed using 20% piperidine in DMF. Coupling reactions were performed using Fmoc-amino acid/HBTU/HOBt/DIEA/resin in molar ratio of 3/3/3/9/1. The coupling and deprotection reactions were checked by the Kaiser test. Peptide removal from the resin and the removal of side chain protection groups were performed using 95% TFA with 0.3% TES treatment. The synthesis of RGD-OMe was prepared by classical solution procedure using mixed anhydride coupling reactions. The final products were purified by gel filtration on a Sephadex G-10 column. The peptide purity was monitored on a RP-HPLC column AtlantisTMdC18, 4.6×150 mm, particle size 5μm, mobile phase: acetonitrile/deionised water 40/60 (v/v), at 25°C, flow rate: 1ml/min, and UV detection - 206 nm. The correct molecular masses were confirmed by ESI-MS. For R(NO2)GD, GF - C12H21O7N7, [MH]+ calculated -375.34, [MH]+ observed -375.36; CavGD, GF - C11H21O6N6, [MH]+ calculated -333.32, [MH]+ observed -333.19; RGD-OMe, GF - C13H24O5N6, [MH]+ calculated -344.37, [MH]+ observed -344.59.
Cell cultures
The 3T3 (standard mouse embryonic fibroblast cell line), MCF-7 (human breast cancer cell line) and HepG2 (human liver hepatocellular carcinoma cell line) cells were cultured in Dulbecco Modified Eagle’s medium (DMEM) (Gibco, Austria) supplemented with 10% fetal bovine serum (Gibco, Austria), 100 U/ml penicillin (Lonza, Belgium) and 0.1 mg/ml streptomycin (Lonza, Belgium) under a humidified 5% CO2 atmosphere at 37°C. Plastic flasks supplied by Greiner, Germany, were used to grow the cells. Cells were trypsinized using Trypsin-EDTA (FlowLab, Australia) when they reached approximately 80% confluence. For experiments the cells in exponential phase of growth after treatment with Trypsin-EDTA were seeded into 96-well plates (Greiner, Germany) in a concentration 2x104 cells/well. 24 hours incubation post seeding (under a humidified 5% CO2 atmosphere at 37°C) allowed the cells to attach to the wells.
Cytotoxicity assay
The cultivated cells were treated with RGD and its analogues (2-4) in a wide concentration range (2 - 0.015 mM). Untreated cells were used as controls. Empty wells were blank controls. Cytotoxicity was measured by colorimetric assay based on tetrasolium salt MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (Sigma Chemical Co.). The MTT assay is based on the protocol first described by Mossman (1983). In this assay, living cells reduce the yellow MTT to insoluble purple formazan crystals. The peptides were dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO in samples did not affect the viability of the cells. The assay was performed 24 hours after treatment with the amino acid analogues. For this purpose, MTT solution was prepared at 5 mg/ml in PBS and was filtered through a 0.2 µm filter. Then 1 ml of MTT solution was added to 15 ml DMEM and 100 µl of this solution were added into each well, including the cell free blank wells. Then the plates were further incubated for 3 hours to allow MTT to be metabolized and the supernatant was removed. 100 µl/well DMSO/etanol (1/1) was added. The plates were placed in a microtitre-plate shaker for 10 min at room temperature to thoroughly mix the purple formazan into the solvent. ELIZA plate reader (TECAN, Sunrise TM, Grodig/Sazburg, Austria) was used for reading the results. Optical density (OD) was determined at a wavelength of 540 nm and a reference wavelength of 620 nm. Cell cytotoxicity determined by MTT assay was expressed as per cent of dead cells:
% cytotoxicity = (1 – (OD sample – OD blank control)/(OD control – OD blank control)) x 100
PrizmaPlot.4 (ANOVA-test) was used for statistical analysis.
References
- Ung P, Winkler DA. Tripeptide Motifs in Biology: Targets for Peptidomimetic Design J Med Chem 2011; 54:1111-1125
Reference Link - Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science 1987; 238: 491-497.
Reference Link - Sasaki M, Kleinman HK, Huber H, Deutzmann R, YamadaY. Laminin, a multidomain protein. The A chain has a unique globular domain and homology with the basement membrane proteoglycan and the laminin B chains. J Biol Chem 1988; 263: 16536-16544.
- Humphries M J, Olden K, Yamada KM. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science 1986; 233: 467-470.
Reference Link - Saiki I, Murata J, Iida J, Sakurai T, Nishi N, Matsuno K, Azuma I. Antimetastatic effects of synthetic polypeptides containing repeated structures of the cell adhesive Arg-Gly-Asp (RGD) and Tyr-Ile-Gly-Ser-Arg (YIGSR) sequences. Br J Cancer 1989; 60: 722-728.
Reference Link - Saiki I, Murata J, Makabe T, Nishi N, Tokura S, Azuma, I. Inhibition of tumor angiogenesis by a synthetic cell-adhesive polypeptide containing the Arg-Gly-Asp (RGD) sequence of fibronectin, poly(RGD). Jpn J Cancer Res 1990; 81: 668-675.
Reference Link - Saito N, Mitsuhashi M, Hayashi T, Narumo C, Nagata H, Soyama K, Kameoka S, Harumiya S, Fujimoto D. Inhibition of hepatic metastasis in mice treated with cell-binding domain of human fibronectin and angiogenesis inhibitor TNP-470. Int J Clin Oncol 2001; 6: 215-220.
Reference Link - Murata J, Saiki I, Ogawa R, Nishi N, Tokura S, Azuma I. Molecular properties of poly(RGD) and its binding capacities to metastatic melanoma cells. Int J Pept Protein Res 1991; 38: 212-217.
Reference Link - Sogabe T, Nishimura S, Chung YS, Sowa M. Role of tripeptide Arg-Gly-Asp (RGD) on adhesiveness of human pancreatic cancer cell, PANC-1 to extracellular matrix. Nippon Rinsho 1995; 53: 1648-1652.
- Meyer A, Auernheimer J, Modlinger A, Kessler H. Targeting RGD recognizing integrins: drug development, biomaterial research, tumor imaging and targeting. Curr Pharm Des 2006; 12:2723-2747.
Reference Link - Yamamoto, Y., Tsutsumi, Y. and Mayumi, T. Molecular design of bioconjugated cell adhesion peptide with a water-soluble polymeric modifier for enhancement of antimetastatic effect. Curr Drug Targets 2002; 3: 123-130.
Reference Link - Tsuchiya Y, Sawada S, Tsukada K, Saiki I. A new pseudo-peptide of Arg-Gly-Asp (RGD) inhibits intrahepatic metastasis of orthotopically implanted murine hepatocellular carcinoma. Int J Oncol 2002; 20: 319-324.
- Saiki I, Yoneda J, Igarashi, Y, Aoki M, Kusunose N, Ono K. Azuma I. Antimetastatic activity of polymeric RGDT peptides conjugated with poly(ethylene glycol). Jpn J Cancer Res 1993; 84: 558-565.
Reference Link - Fujii H, Nishikawa N, Komazawa H, Orikasa A, Ono M, Itoh I, Murata J, Azuma I, Saiki I. Inhibition of tumor invasion and metastasis by peptidic mimetics of Arg-Gly Asp (RGD) derived from the cell recognition site of fibronectin. Oncol Res 1996; 8: 333-342.
- Mukhopadhyay S, Barn?s CM, Haskel A, Short SM, Barnes KR, Lippard SJ. Conjugated Platinum(IV)-Peptide Complexes for Targeting Angiogenic Tumor Vasculature. Bioconjugate Chem. 2008; 19: 39-49.
Reference Link - Raha S, Paunesku T, Woloschak G. Peptide-mediated cancer targeting of nanoconjugates. WIREs Nanomedicine and Nanobiotechnology 2011; 3: 269-281.
Reference Link - Wang W, Borchardt R T, Wang B. Orally active peptidomimetic RGD analogs that are glycoprotein IIb/IIIa antagonists. Curr Med Chem. 2000; 7:437-453.
- Pajpanova T, Georgiev K, Dzimbova T, Georgieva M, Staneva D, Miloshev G. In vitro Assessment of the Cytotoxic Effects of Novel RGD-Mimetics, in Peptides: Building Bridges Proceedings of the 22nd American Peptide Symposium (Lebl M. ed.), 2011, Prompt Scientific Publishing, San Diego, USA, pp. 190-191.
- Bence AK, Worthen DR, Adams VR, Crooks PA. The antiproliferative and immunotoxic effects of L-canavanine and L-canaline. Anticancer Drugs 2002; 13: 313-320.
Reference Link - Miersh J, Grancharov K, Pajpanova T, Neumann D, Tabakova S, Stoev S, Krauss G-J, Golovinsky E. Synthesis and biological activity of canavanine hydrazide derivatives. Amino Acids 2000; 18: 41-59.
Reference Link - Dzimbova T, Iliev I, Georgiev K, Detcheva R, Balacheva A, Pajpanova T. In vitro assessment of the cytotoxic effects of sulfo-arginine analogues and their hydrazide derivatives in 3T3 and HepG2 cells. Biotechnol & Biotechnol Eq 2012; 26: 180-184.
Reference Link