The role of p53 in the acute response to DNA damage
The transcription factor p53 is activated in response to a wide range of cellular stresses, including DNA damage (such as that induced by anti-cancer therapeutics), aberrant growth signals (e.g. as a consequence of oncogene activation), hypoxia, reactive oxygen species (ROS) and nucleotide depletion [
Maintenance of genomic integrity is critical for cell survival (and in the germline for propagation of the species). Cells have therefore evolved complex systems for the recognition and repair of DNA damage. Recognition of DNA lesions, such as double strand breaks (e.g. induced by γ-irradiation), replication stress induced by hyper-proliferation (particularly important in the context of cancer) or by stalling of replication forks during cell cycling, can lead to activation and recruitment of the kinases ATM, ATR, Chk1 and Chk2 to sites of damage. ATM and ATR are serine/threonine kinases that are directly activated in response to DNA damage and function to phosphorylate key substrates, including p53, MDM2 and the downstream kinases Chk1/Chk2 [
Cell cycle arrest and cellular senescence
In response to DNA damage p53 can induce arrest of cellular proliferation. This can serve two functions: allowing cells time to repair their damaged DNA and blocking damaged cells from proliferating and thereby propagating aberrant DNA changes. This growth arrest can be transient or, in the case of cellular senescence, permanent.
The best characterised mediator of p53-induced cell growth arrest is p21 (also called WAF1/CIP1). p21 was originally identified as a p53-responsive gene in a human glioblastoma cell carrying an inducible wild-type (wt) p53 expression construct [
Interestingly, p21 has also been suggested to function as an inhibitor of DNA damage induced cell death. Loss of p21 was shown to enhance the sensitivity of HCT116 colon cancer cells to daunomycin [
Additional p53 target genes, induced in response to DNA damage, have been implicated in the control of cell cycle progression at the G1-S and also other checkpoints. For example, MEFs and intestinal epithelial cells lacking the transmembrane tyrosine phosphatase, Ptprv, failed to arrest at the G1-S boundary after γ-irradiation [
In response to DNA damage cells can also undergo irreversible growth arrest, a process called cellular senescence. Cells undergoing senescence become irreversibly halted in the G1 phase of the cell cycle but remain metabolically active [
Apoptosis
In certain cells, particularly hematopoietic ones and intestinal epithelial cells, DNA damage will preferentially induce apoptosis, a form of programmed cell suicide responsible for the removal of unwanted or damaged cells from multi-cellular organisms [
A role for p53 in apoptosis signalling was first indicated in studies that found that enforced expression of wt p53 induced characteristic features of apoptotic cell death [
The intrinsic (also called “mitochondrial”, “Bcl-2 regulated” or “stress induced”) apoptotic pathway can be activated by developmental cues or diverse cell stressors including cytokine deprivation or DNA damage. Life versus death decisions in this pathway are controlled by the Bcl-2 protein family, which can be divided into three functional sub-groups: the pro-survival Bcl-2 family members (Bcl-2, Bcl-XL, Bcl-W, Mcl-1 and A1), the pro-apoptotic BH3-only proteins (Bad, Bid, Bik, Bim, Bmf, Hrk, Puma and Noxa) and the multi-BH domain pro-apoptotic Bcl-2 family members (Bak, Bax and possibly Bok) [
A direct link between p53 and the intrinsic apoptotic pathways was identified when it was found that the pro-apoptotic Bcl-2 family members Puma, Noxa and Bax are direct transcriptional targets of p53. The puma gene has a conserved p53 binding site (consensus sequence) within its first intron, and mutation of this site was shown to abrogate p53-dependent expression of Puma [
Noxa, another BH3-only protein, is also a direct p53 target [
So, what are the roles of other p53 targets that have been implicated as effectors of apoptosis? In the case of PERP, PIG3 and certain others [
Several reports have indicated that p53, activated by DNA damage, kills cells through stimulation of the “death receptor” (also called “extrinsic”) apoptotic pathway. In this pathway (which operates largely in parallel to the “intrinsic apoptotic” pathway [
Several studies have indicated that p53 may trigger apoptosis via a non-transcriptional mechanism. It was reported that in response to γ-irradiation and certain other stresses, p53 can shuttle to the outer mitochondrial membrane where it directly interacts with members of the Bcl-2 protein family to cause MOMP and consequent activation of the caspase cascade. Curiously, p53 was shown to bind to anti-apoptotic Bcl-XL as well as pro-apoptotic Bax and Bak to facilitate MOMP [
In conclusion, in response to DNA damage and certain other stress conditions (e.g. hypoxia, ROS) the tumour suppressor p53 is activated through complex post-translational mechanisms and then transcriptionally upregulates target genes, which then mediate cellular responses, including cell cycle arrest, apoptosis and coordination of DNA repair.
However, while we have a strong understanding of how these effector processes are orchestrated, questions remain as to how cell fate is determined after p53 activation, namely what determines whether in response to DNA damage a cell will undergo growth arrest and continue to survive or die? The choice between life and death is likely to be modulated by a wide range of factors, including the type and strength of the stress applied (although to date this has been only poorly correlated to cellular fate), differences in the inducibility of initiators of apoptosis or cell senescence, influence of other (p53-independent) signalling pathways that are activated, differences in the basal expression of pro-survival proteins and perhaps also factors that function downstream to limit activity of the effector pathways, such as inhibition of Puma induction by Slug [
The role of p53 in tumour suppression
Although originally identified as a proto-oncogene, it was soon revealed that p53 exhibited tumour suppressive actions. In particular, p53 was shown to inhibit E1A and Ras-induced transformation of rat fibroblasts [
While the function of p53 in mediating cellular responses after acute and extensive DNA damage (e.g. in response to γ-irradiation or treatment with etoposide) is firmly established, the manner in which p53 suppresses the development of cancer is less well understood. Stabilisation and activation of p53 is known to occur in response to expression of certain onco-proteins, such as c-Myc or mutant Ras, which trigger transcription of the tumour suppressors p14ARF (humans) or p19ARF (mouse) [
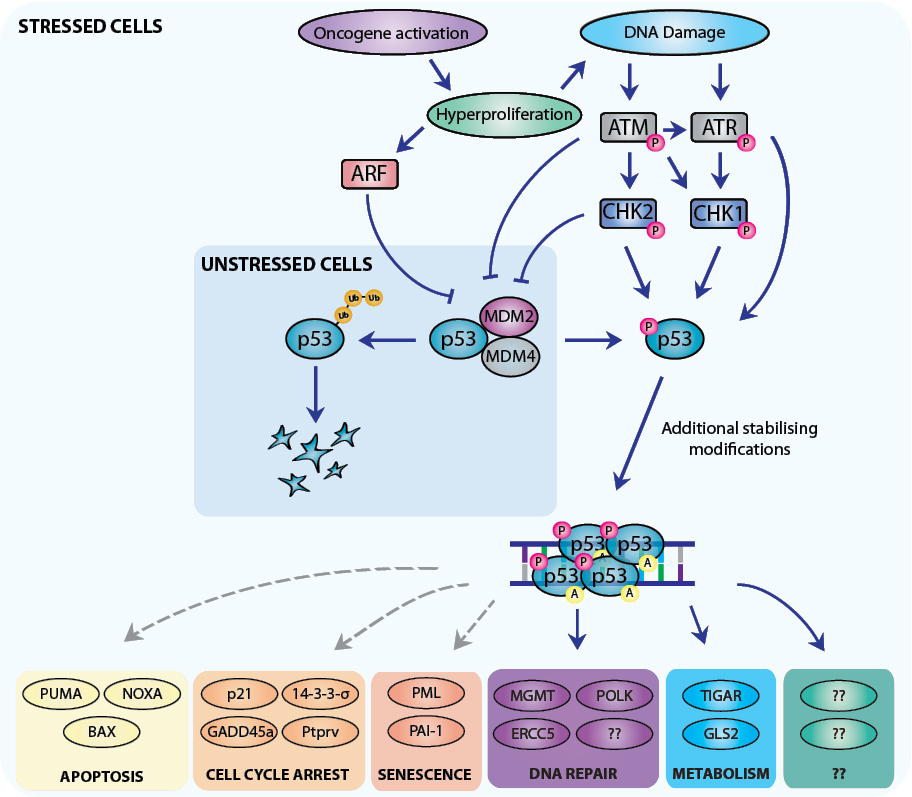
Given the critical roles of apoptosis, G1-S boundary cell cycle arrest and senescence in the p53-regulated responses to acute DNA damage in non-transformed cells, it has been widely proposed that these same processes must also be essential for p53-mediated tumour suppression [
Interesting insight into the impact of restoration of p53 function in established malignant tumours that had developed as a result of loss or mutation of p53 came from studies of Gerard Evan and colleagues. They generated p53 knock-in mice in which the coding sequences of wt Trp53 were fused to the coding region of a tamoxifen-sensitive version of the oestrogen oestrogen receptor (p53ERTAM mice). Expression of p53 in these mice could therefore be switched on or off in vivo by the administration of 4-hydroxy-tamoxifen (4-OHT) [
In another study, Jacks and colleagues [
These studies provide evidence that apoptosis and/or senescence play a critical role in p53-mediated regression of established tumours, and were widely thought to provide insight into the mechanism by which p53 suppresses the initiation of tumour formation. Unexpectedly, however, mice lacking single or multiple target genes that are essential for p53-mediated induction of apoptosis, cell cycle arrest or senescence, such as p21-/-, puma-/-, noxa-/- and puma-/-noxa-/- mice, do not develop tumours (unlike the p53-deficient or p53 mutant mice) [
Evans and colleagues, utilising the aforementioned p53ERTAM knock-in mice probed the requirement for the acute DNA damage response triggered by p53 in the suppression of tumour development [
In order to dissect the specific requirements for the different transactivation domains in p53 for tumour suppression, Attardi and colleagues generated a panel of p53 conditional knock-in mice bearing mutations in specific residues within these regions [
Data from another p53 mutant knock-in mouse strain however appears to rule out a critical role for cellular senescence in p53-mediated tumour suppression. In order to define the role for acetylation in modulating p53 function, Gu and colleagues generated a p53 knock-in mouse strain bearing mutations in three conserved residues within the DNA binding domain of p53 (K117R, K161R and K162R: p533KR mice) that are acetylated in response to DNA damage [
Although these studies constitute a substantial advance in our knowledge of how p53 mediates tumour suppression, an important caveat of the studies of both of the aforementioned p53 mutant mouse strains is that the expression of the critical effectors of apoptosis, cell cycle arrest and senescence (i.e. Puma and Noxa as well as p21, respectively) was only reduced but not abrogated. It therefore remained possible that residual p53-mediated expression of these target genes might be sufficient to suppress tumour development (although this was not sufficient to induce apoptosis, cell cycle arrest and senescence in response to acute DNA damage). In order to resolve this issue, we have recently generated mice that lack all of the critical effectors of p53-mediated apoptosis (Puma and Noxa) and G1-S cell cycle arrest as well as cellular senescence (p21) and investigated their predisposition to cancer. Consistent with previous studies of mice lacking Puma [
These data do not, however, exclude that p53-induced apoptosis, cell cycle arrest and/or senescence may contribute to p53-mediated tumour suppression in the context of certain oncogenic driver mutations. Indeed, as discussed earlier, the loss of Puma or the combined loss of Puma and Noxa accelerated Myc-induced lymphoma development [
So the question arises, which effector processes controlled by p53 are critical for tumour suppression? As mentioned above, in their studies with the p533KR/3KR mice, Gu and colleagues concluded that coordination of metabolism might be critical [
While coordination of metabolism represents a credible and interesting possibility to explain how p53 suppresses tumour formation, other known and possibly also some unknown p53 effector processes may be equally or even more critical. Interestingly, we found that upon γ-irradiation, cells from p53-/- animals (which are tumour-prone) displayed impaired induction of genes implicated in DNA repair and a trend towards abnormally increased persistence of γH2AX foci (a marker of detection of double-strand DNA breaks and initiation of their repair) [
Another intriguing idea is that p53 may also function as a guardian of normal cellular stresses, in addition to its defined roles in responses to acute stresses. In healthy cells p53 is constitutively expressed, albeit restrained at low levels. Accordingly, constitutive expression of p53 would allow for its transient activation in response to normal cellular stresses such as, DNA strand breaks that occur during replication or transient changes in cellular metabolites. Such transient p53 activation would be predicted to be insufficient to activate apoptosis or cellular senescence, either because these stresses induce different overall transcriptional programs or simply due to the transient nature of the p53 response in this context (e.g. to elicit apoptosis, Puma/Noxa levels must increase sufficiently to overcome the protective effects of the pro-survival Bcl-2 family members present within a given cell). Such transient p53 activation, although at low levels, might be sufficient to counter early tumourigenic events, such as oncogene activation.
Regardless, it is apparent that much remains unknown about how p53 mediates tumour suppression, and so, the search for the p53 target genes and the processes they regulate that are critical to protect us from developing cancer continues. Much work remains to validate the function of the newly discovered candidate genes implicated as critical for tumour suppression in the aforementioned studies (e.g. through use of gene-targeting strategies on targets identified by Attardi et al [
Conclusions
To conclude, p53 imposes a critical barrier against the development of cancer. However, the mechanisms by which p53 mediates tumour suppression remain elusive. Surprisingly, it appears that suppression of spontaneous tumour formation by p53 utilises distinct effector processes from those that are critical for the cellular responses to acute DNA damage. Experiments utilising p53 mutant knock-in mice or gene-targeted knock-out mice lacking well-characterised p53 effector genes have demonstrated that induction of apoptosis, cell cycle arrest and cellular senescence, even in combination, appear largely dispensable for the ability of p53 to suppress spontaneous tumour development. Instead, p53 must mobilise currently underappreciated processes, such as coordination of DNA repair, control of metabolic adaptation or perhaps even currently unknown processes to suppress cancer formation. Defining the effector processes that are critical for p53-mediated tumour suppression and how the signalling pathways responsible for these processes are triggered by oncogenic events remain critical goals of future research. Detailed understanding of these processes will have great potential to aid in the development of novel strategies for cancer therapy and possibly even cancer prevention.
Acknowledgements
The authors thank Drs JM Adams, S Cory, Y Haupt and L O’Connor and all members of the Molecular Genetics of Cancer Division for scientific collaborations and discussions. Work in the authors’ laboratory was supported by the National Health and Medical Research Council of Australia (Program Grant 101670, Project Grant 1046010 and 637326), Australia Fellowship (Fellowship Grant 461299), SPRF Fellowship (Fellowship Grant 1020363), the Leukemia and Lymphoma Society (SCOR grant #7413), the Cancer Council Victoria (PhD fellowship to LV), project grants to AS (Project Grant 1052309), the National Health and Medical Research Council of Australia/Juvenile Diabetes Research Foundation (Project Grant 466658) and operational infrastructure grants through the Australian Government (IRISS) and the Victorian State Government (OIS).
References
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell 1997; 88: 323-331.
Reference Link - Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M, et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO Journal 1996; 15: 5349-5357.
- Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett 1997; 420: 25-27.
Reference Link - Barak Y, Juven T, Haffner R, Oren M. mdm2 expression is induced by wild type p53 activity. EMBO J 1993; 12: 461-468.
- Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 1996; 274: 948-953.
Reference Link - Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature 1997; 387: 296-299.
Reference Link - Kubbutat MHG, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature 1997; 387: 299-303.
Reference Link - Kruse JP, Gu W. Modes of p53 regulation. Cell 2009; 137: 609-622.
Reference Link - Loughery J, Meek D. Switching on p53: an essential role for protein phosphorylation? Biodiscovery 2013; 8: 1.
- Kim ST, Lim DS, Canman CE, Kastan MB. Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem 1999; 274: 37538-37543.
Reference Link - Tibbetts RS, Brumbaugh KM, Williams JM, Sarkaria JN, Cliby WA, Shieh S-Y, et al. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes and Development 1999; 13: 152-157.
Reference Link - O'Neill T, Dwyer AJ, Ziv Y, Chan DW, Lees-Miller SP, Abraham RH, et al. Utilization of oriented peptide libraries to identify substrate motifs selected by ATM. J Biol Chem 2000; 275: 22719-22727.
Reference Link - Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003; 421: 499-506.
Reference Link - Shieh S-Y, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 1997; 91: 325-334.
Reference Link - Banin S, Moyal L, Shieh S-Y, Taya Y, Anderson CW, Chessa L, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 1998; 281: 1674-1677.
Reference Link - Canman CE, Lim D-S, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 1998; 281: 1677-1679.
Reference Link - Khanna KK, Keating KE, Kozlov S, Scott S, Gatei M, Hobson K, et al. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat Genet 1998; 20: 398-400.
Reference Link - Ashcroft M, Kubbutat MH, Vousden KH. Regulation of p53 function and stability by phosphorylation. Mol Cell Biol 1999; 19: 1751-1758.
- Dumaz N, Meek DW. Serine15 phosphorylation stimulates p53 transactivation but does not directly influence interaction with HDM2. EMBO J 1999; 18: 7002-7010.
Reference Link - Khosravi R, Maya R, Gottlieb T, Oren M, Shiloh Y, Shkedy D. Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl Acad Sci U S A 1999; 96: 14973-14977.
Reference Link - Maya R, Balass M, Kim ST, Shkedy D, Leal JF, Shifman O, et al. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev 2001; 15: 1067-1077.
Reference Link - Bartek J, Falck J, Lukas J. CHK2 kinase--a busy messenger. Nat Rev Mol Cell Biol 2001; 2: 877-886.
Reference Link - McGowan CH. Checking in on Cds1 (Chk2): A checkpoint kinase and tumor suppressor. Bioessays 2002; 24: 502-511.
Reference Link - Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer 2002; 2: 594-604.
Reference Link - el-Deiry WS, Kern SE, Pietenpol JA, Kinzler KW, Vogelstein B. Definition of a consensus binding site for p53. Nat Genet 1992; 1: 45-9.
Reference Link - Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 2008; 9: 402-412.
Reference Link - El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell 1993; 75: 817-825.
Reference Link - Gu Y, Turck CW, Morgan DO. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature 1993; 366: 707-710.
Reference Link - Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993; 75: 805-816.
Reference Link - Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashl R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature 1993; 366: 701-704.
Reference Link - Rousseau D, Cannella D, Boulaire J, Fitzgerald P, Fotedar A, Fotedar R. Growth inhibition by CDK-cyclin and PCNA binding domains of p21 occurs by distinct mechanisms and is regulated by ubiquitin-proteasome pathway. Oncogene 1999; 18: 4313-4325.
Reference Link - Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995; 82: 675-684.
Reference Link - Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 1995; 377: 552-557.
Reference Link - Javelaud D, Besançon F. Inactivation of p21WAF1 sensitizes cells to apoptosis via an increase of both p14ARF and p53 levels and an alteration of the Bax/Bcl-2 ratio. JBC 2002; 277: 37949-37954.
Reference Link - Xia M, Knezevic D, Vassilev LT. p21 does not protect cancer cells from apoptosis induced by nongenotoxic p53 activation. Oncogene 2011; 30: 346-355.
Reference Link - Doumont G, Martoriati A, Beekman C, Bogaerts S, Mee PJ, Bureau F, et al. G1 checkpoint failure and increased tumor susceptibility in mice lacking the novel p53 target Ptprv. EMBO J 2005; 24: 3093-3103.
Reference Link - Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, Walsh WV, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 1992; 71: 587-597.
Reference Link - Zhan Q, Bae I, Kastan MB, Fornace AJ, Jr. The p53-dependent gamma-ray response of GADD45. Cancer Res 1994; 54: 2755-2760.
- Wang XW, Zhan Q, Coursen JD, Khan MA, Kontny HU, Yu L, et al. GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci U S A 1999; 96: 3706-3711.
Reference Link - Zhan Q, Antinore MJ, Wang XW, Carrier F, Smith ML, Harris CC, et al. Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene 1999; 18: 2892-2900.
Reference Link - Jin S, Antinore MJ, Lung FD, Dong X, Zhao H, Fan F, et al. The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J Biol Chem 2000; 275: 16602-16608.
Reference Link - Laronga C, Yang HY, Neal C, Lee MH. Association of the cyclin-dependent kinases and 14-3-3 sigma negatively regulates cell cycle progression. J Biol Chem 2000; 275: 23106-23112.
Reference Link - Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene 2001; 20: 1803-1815.
Reference Link - Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995; 92: 9363-9367.
Reference Link - Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997; 88: 593-602.
Reference Link - Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 1997; 91: 649-659.
Reference Link - Dimri GP, Itahana K, Acosta M, Campisi J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol Cell Biol 2000; 20: 273-285.
Reference Link - Harvey M, Sands AT, Weiss RS, Hegi ME, Wiseman RW, Pantazis P, et al. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene 1993; 8: 2457-2467.
- Ferbeyre G, de Stanchina E, Querido E, Baptiste N, Prives C, Lowe SW. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev 2000; 14: 2015-2027.
- Pearson M, Carbone R, Sebastiani C, Cioce M, Fagioli M, Saito S, et al. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 2000; 406: 207-210.
Reference Link - Comi P, Chiaramonte R, Maier JA. Senescence-dependent regulation of type 1 plasminogen activator inhibitor in human vascular endothelial cells. Exp Cell Res 1995; 219: 304-308.
Reference Link - Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res 1994; 211: 90-98.
Reference Link - Tahara H, Sato E, Noda A, Ide T. Increase in expression level of p21sdi1/cip1/waf1 with increasing division age in both normal and SV40-transformed human fibroblasts. Oncogene 1995; 10: 835-840.
- Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci U S A 1996; 93: 13742-13747.
Reference Link - Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 1961; 25: 585-621.
Reference Link - Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990; 345: 458-460.
Reference Link - Di Leonardo A, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev 1994; 8: 2540-2551.
Reference Link - Tsukada T, Tomooka Y, Takai S, Ueda Y, Nishikawa S-i, Yagi T, et al. Enhanced proliferative potential in culture of cells from p53-deficient mice. Oncogene 1993; 8: 3313-3322.
- Jones SN, Sands AT, Hancock AR, Vogel H, Donehower LA, Linke SP, et al. The tumorigenic potential and cell growth characteristics of p53-deficient cells are equivalent in the presence or absence of Mdm2. Proc Natl Acad Sci U S A 1996; 93: 14106-14111.
Reference Link - Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J, et al. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene 1999; 18: 4808-4818.
Reference Link - Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 2002; 109: 335-346.
Reference Link - Pantoja C, Serrano M. Murine fibroblasts lacking p21 undergo senescence and are resistant to transformation by oncogenic Ras. Oncogene 1999; 18: 4974-4982.
Reference Link - de Stanchina E, Querido E, Narita M, Davuluri RV, Pandolfi PP, Ferbeyre G, et al. PML is a direct p53 target that modulates p53 effector functions. Mol Cell 2004; 13: 523-535.
Reference Link - Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol 2006; 8: 877-884.
Reference Link - Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med 2009; 361: 1570-1583.
Reference Link - Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer 1972; 26: 239-257.
Reference Link - Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Ann Rev Biochem 2000; 69: 217-245.
Reference Link - Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2002; 2: 965-975.
Reference Link - Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980; 284: 555-556.
Reference Link - Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998; 281: 1312-1316.
Reference Link - Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 1991; 352: 345-347.
Reference Link - Shaw P, Bovey R, Tardy S, Sahli R, Sordat B, Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci USA 1992; 89: 4495-4499.
Reference Link - Johnson P, Chung S, Benchimol S. Growth suppression of Friend virus-transformed erythroleukemia cells by p53 protein is accompanied by hemoglobin production and is sensitive to erythropoietin. Molecular and Cellular Biology 1993; 13: 1456-1462.
- Ramqvist T, Magnusson KP, Wang Y, Szekely L, Klein G, Wiman KG. Wild-type p53 induces apoptosis in a Burkitt lymphoma (BL) line that carries mutant p53. Oncogene 1993; 8: 1495-1500.
- Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, et al. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 1993; 362: 849-852.
Reference Link - Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 1993; 362: 847-849.
Reference Link - Strasser A, Harris AW, Jacks T, Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell 1994; 79: 329-339.
Reference Link - Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, et al. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Research 1994; 54: 614-617.
- Strasser A, Harris AW, Cory S. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 1991; 67: 889-899.
Reference Link - Wang Y, Szekely L, Okan I, Klein G, Wiman KG. Wild-type p53-triggered apoptosis is inhibited by bcl-2 in a v-myc-induced T-cell lymphoma line. Oncogene 1993; 8: 3427-3431.
- Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell 2000; 6: 1389-1399.
Reference Link - Rathmell JC, Lindsten T, Zong W-X, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nature Immunology 2002; 3: 932-939.
Reference Link - Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47-59.
Reference Link - Ke F, Voss A, Kerr JB, O'Reilly LA, Tai L, Echeverry N, et al. BCL-2 family member BOK is widely expressed but its loss has only minimal impact in mice. Cell Death Differ 2012; 19: 915-925.
Reference Link - Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2002; 2: 183-192.
Reference Link - Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, et al. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell 2004; 16: 807-818.
Reference Link - Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol 2006; 8: 1348-1358.
Reference Link - Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 2007; 315: 856-859.
Reference Link - Han J, Flemington C, Houghton AB, Gu Z, Zambetti GP, Lutz RJ, et al. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci U S A. 2001; 98: 11318-11323.
Reference Link - Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 2001; 7: 683-694.
Reference Link - Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 2001; 7: 673-682.
Reference Link - Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 2003; 4: 321-328.
Reference Link - Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003; 302: 1036-1038.
Reference Link - Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, Coultas L, et al. BH3-only proteins Puma and Bim are rate-limiting for {gamma} -radiation and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 2005; 106: 4131-4138.
Reference Link - Erlacher M, Laabi V, Manzl C, Bock G, Tzankov A, Haecker G, et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. Journal of Experimental Medicine 2006; 203: 2939-2951.
Reference Link - Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the bcl-2 family and candidate mediator of p53-induced apoptosis. Science 2000; 288: 1053-1058.
Reference Link - Schuler M, Maurer U, Goldstein JC, Breitenbucher F, Hoffarth S, Waterhouse NJ, et al. p53 triggers apoptosis in oncogene-expressing fibroblasts by the induction of Noxa and mitochondrial Bax translocation. Cell Death and Differentiation 2003; 10: 451-460.
Reference Link - Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev 2003; 17: 2233-2238.
Reference Link - Naik E, Michalak EM, Villunger A, Adams JM, Strasser A. UV-radiation triggers apoptosis of fibroblasts and skin keratinocytes mainly via the BH3-only protein Noxa. Journal of Cell Biology 2007; 176: 415-424.
Reference Link - Michalak EM, Villunger A, Adams JM, Strasser A. In several cell types the tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ 2008; 15: 1019-1029.
Reference Link - Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 2005; 17: 393-403.
Reference Link - Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 Domains of BH3-Only Proteins Differentially Regulate Bax-Mediated Mitochondrial Membrane Permeabilization Both Directly and Indirectly. Mol Cell 2005; 17: 525-535.
Reference Link - Letai A, Bassik M, Walensky L, Sorcinelli M, Weiler S, Korsmeyer S. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2002; 2: 183-192.
Reference Link - Zhang Y, Xing D, Liu L. PUMA promotes Bax translocation by both directly interacting with Bax and by competitive binding to Bcl-X L during UV-induced apoptosis. Mol Biol Cell 2009; 20: 3077-3087.
Reference Link - Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature 1997; 389: 300-305.
Reference Link - Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, et al. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes and Development 2000; 14: 704-718.
- Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, et al. Regulation of PTEN transcription by p53. Molecular Cell 2001; 8: 317-325.
Reference Link - Strasser A, Harris AW, Huang DCS, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J 1995; 14: 6136-6147.
- Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity 2009; 30: 180-192.
Reference Link - Owen-Schaub LB, Zhang W, Cusack JC, Angelo LS, Santee SM, Fujiwara T, et al. Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Molecular and Cellular Biology 1995; 15: 3032-3040.
- Wu GS, Burns TF, McDonald ER, III, Jiang W, Meng R, Krantz ID, et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nature Genetics 1997; 17: 141-143.
Reference Link - Bennett M, Macdonald K, Chan S-W, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 1998; 282: 290-293.
Reference Link - Sheikh MS, Huang Y, Fernandez-Salas EA, El-Deiry WS, Friess H, Amundson S, et al. The antiapoptotic decoy receptor TRID/TRAIL-R3 is a p53-regulated DNA damage-inducible gene that is overexpressed in primary tumors of the gastrointestinal tract. Oncogene 1999; 18: 4153-4159.
Reference Link - Lin Y, Ma W, Benchimol S. Pidd, a new death-domain-containing protein, is induced by p53 and promotes apoptosis. Nature Genetics 2000; 26: 124-127.
- Meng RD, McDonald ER, 3rd, Sheikh MS, Fornace AJ, Jr., El-Deiry WS. The TRAIL decoy receptor TRUNDD (DcR2, TRAIL-R4) is induced by adenovirus-p53 overexpression and can delay TRAIL-, p53-, and KILLER/DR5-dependent colon cancer apoptosis. Mol Ther 2000; 1: 130-144.
Reference Link - Takimoto R, El-Deiry WS. Wild-type p53 transactivates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene 2000; 19: 1735-1743.
Reference Link - Guan B, Yue P, Clayman GL, Sun SY. Evidence that the death receptor DR4 is a DNA damage-inducible, p53-regulated gene. J Cell Physiol 2001; 188: 98-105.
Reference Link - Ruiz de Almodovar C, Ruiz-Ruiz C, Rodriguez A, Ortiz-Ferron G, Redondo JM, Lopez-Rivas A. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) decoy receptor TRAIL-R3 is up-regulated by p53 in breast tumor cells through a mechanism involving an intronic p53-binding site. J Biol Chem 2004; 279: 4093-4101.
Reference Link - Newton K, Harris AW, Bath ML, Smith KGC, Strasser A. A dominant interfering mutant of FADD/Mort1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO Journal 1998; 17: 706-718.
Reference Link - Newton K, Strasser A. Ionizing radiation and chemotherapeutic drugs induce apoptosis in lymphocytes in the absence of fas or FADD/MORT1 signaling: implications for cancer therapy. Journal of Experimental Medicine 2000; 191: 195-200.
Reference Link - O'Connor L, Strasser A. Fas, p53, and apoptosis. Science 1999; 284: 1430.
Reference Link - Smith KGC, Strasser A, Vaux DL. CrmA expression in T lymphocytes of transgenic mice inhibits CD95 (Fas/APO-1)-transduced apoptosis, but does not cause lymphadenopathy or autoimmune disease. EMBO Journal 1996; 15: 5167-5176.
- Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 1998; 9: 267-276.
Reference Link - Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 1998; 279: 1954-1958.
Reference Link - Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 1998; 392: 296-300.
Reference Link - Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au B, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes and Development 2003; 17: 883-895.
Reference Link - Kang TB, Oh GS, Scandella E, Bolinger B, Ludewig B, Kovalenko A, et al. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol 2008; 181: 2522-2532.
- Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. Journal of Biological Chemistry 2000; 275: 16202-16212.
Reference Link - Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003; 11: 577-90.
Reference Link - Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004; 303: 1010-1014.
Reference Link - Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol 2004; 6: 443-450.
Reference Link - Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature 2009; 458: 1127-1130.
Reference Link - Sellins KS, Cohen JJ. Gene induction by g-irradiation leads to DNA fragmentation in lymphocytes. Journal of Immunology 1987; 139: 3199-3206.
- Chao C, Saito S, Kang J, Anderson CW, Appella E, Xu Y. p53 transcriptional activity is essential for p53-dependent apoptosis following DNA damage. EMBO Journal 2000; 19: 4967-4975.
Reference Link - Jimenez GS, Nister M, Stommel JM, Beeche M, Barcarse EA, Zhang XQ, et al. A transactivation-deficient mouse model provides insights into Trp53 regulation and function. Nature Genetics 2000; 26: 37-43.
Reference Link - Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM, et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell 2011; 145: 571-583.
Reference Link - Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, et al. Slug Antagonizes p53-Mediated Apoptosis of Hematopoietic Progenitors by Repressing puma. Cell 2005; 123: 641-653.
Reference Link - Finlay CA, Hinds PW, Levine AJ. The p53 proto-oncogene can act as a suppressor of transformation. Cell 1989; 57: 1083-1093.
Reference Link - Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000; 408: 307-310.
Reference Link - Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol 2007; 8: 275-283.
Reference Link - Malkin D, Li FP, Strong LC, Fraumeni JFJ, Nelson CE, Kim DH, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 1990; 250: 1233-1238.
Reference Link - Srivastava S, Zou ZQ, Pirollo K, Plattner W, Chang EH. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature 1990; 348: 747-749.
Reference Link - Varley JM, Thorncroft M, McGown G, Appleby J, Kelsey AM, Tricker KJ, et al. A detailed study of loss of heterozygosity on chromosome 17 in tumours from Li-Fraumeni patients carrying a mutation to the TP53 gene. Oncogene 1997; 14: 865-871.
Reference Link - Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CAJ, Butel JS, et al. Mice deficient for p53 are developmentally normal but are susceptible to spontaneous tumours. Nature 1992; 356: 215-221.
Reference Link - Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994; 4: 1-7.
Reference Link - Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, et al. Mutant p53 Gain of Function in Two Mouse Models of Li-Fraumeni Syndrome. Cell 2004; 119: 847-860.
Reference Link - Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev 2003; 13: 77-83.
Reference Link - Kamijo T, Weber JD, Zambetti G, Zindy F, Roussel MF, Sherr CJ. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci U S A 1998; 95: 8292-8297.
Reference Link - Pomerantz J, Schreiber-Agus N, Liégeois NJ, Silverman A, Alland L, Chin L, et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 1998; 92: 713-723.
- Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 1998; 92: 725-734.
Reference Link - Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005; 434: 864-870.
Reference Link - Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 2005; 434: 907-913.
Reference Link - Liu G, Parant JM, Lang G, Chau P, Chavez-Reyes A, El-Naggar AK, et al. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet 2004; 36: 63-68.
Reference Link - Christophorou MA, Martin-Zanca D, Soucek L, Lawlor ER, Brown-Swigart L, Verschuren EW, et al. Temporal dissection of p53 function in vitro and in vivo. Nat Genet 2005; 37: 718-726.
Reference Link - Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 1985; 318: 533-538.
Reference Link - Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 2006; 127: 1323-13234.
Reference Link - Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 1990; 348: 331-333.
Reference Link - Garrison SP, Jeffers JR, Yang C, Nilsson JA, Hall MA, Rehg JE, et al. Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol 2008; 28: 5391-5402.
Reference Link - Michalak EM, Jansen ES, Happo L, Cragg MS, Tai L, Smyth GK, et al. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ 2009; 16: 684-696.
Reference Link - Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci U S A 2004; 101: 6164-6169.
Reference Link - Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature 2007; 445: 661-665.
Reference Link - Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007; 445: 656-660.
Reference Link - Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet 2007; 39: 99-105.
Reference Link - Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 2006; 443: 214-217.
Reference Link - Ringshausen I, O'Shea CC, Finch AJ, Swigart LB, Evan GI. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell 2006; 10: 501-514.
Reference Link - Dudgeon C, Kek C, Demidov ON, Saito S, Fernandes K, Diot A, et al. Tumor susceptibility and apoptosis defect in a mouse strain expressing a human p53 transgene. Cancer Res 2006; 66: 2928-2936.
Reference Link - Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM, et al. Distinct p53 Transcriptional Programs Dictate Acute DNA-Damage Responses and Tumor Suppression. Cell 2011; 145: 571-583.
Reference Link - Johnson TM, Hammond EM, Giaccia A, Attardi LD. The p53QS transactivation-deficient mutant shows stress-specific apoptotic activity and induces embryonic lethality. Nat Genet 2005; 37: 145-152.
Reference Link - Jiang D, Brady CA, Johnson TM, Lee EY, Park EJ, Scott MP, et al. Full p53 transcriptional activation potential is dispensable for tumor suppression in diverse lineages. Proc Natl Acad Sci U S A 2011; 108: 17123-17128.
Reference Link - Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 2012; 149: 1269-1283.
Reference Link - Berger SL. Keeping p53 in check: a high-stakes balancing act. Cell 2010; 142: 17-19.
Reference Link - Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell 2008; 133: 612-626.
Reference Link - Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 2006; 126: 107-120.
Reference Link - Valente LJ, Gray DHD, Michalak EM, Pinon-Hofbauer J, Egle A, Scott CL, et al. p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma and Noxa. Cell Reports 2013; 3: 1339-1345.
Reference Link - Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029-1033.
Reference Link - Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci U S A 2010; 107: 7461-7466.
Reference Link - Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci U S A 2010; 107: 7455-7460.
Reference Link - Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res 2004; 64: 2627-2633.
Reference Link - Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011; 11: 85-95.
Reference Link - Lane DP. p53, guardian of the genome. Nature 1992; 358: 15-16.
Reference Link - Scherer SJ, Welter C, Zang KD, Dooley S. Specific in vitro binding of p53 to the promoter region of the human mismatch repair gene hMSH2. Biochem Biophys Res Commun 1996; 221: 722-728.
Reference Link - Liebetrau W, Budde A, Savoia A, Grummt F, Hoehn H. p53 activates Fanconi anemia group C gene expression. Hum Mol Genet 1997; 6: 277-283.
Reference Link - Grombacher T, Eichhorn U, Kaina B. p53 is involved in regulation of the DNA repair gene O6-methylguanine-DNA methyltransferase (MGMT) by DNA damaging agents. Oncogene 1998; 17: 845-851.
Reference Link - Carrier F, Georgel PT, Pourquier P, Blake M, Kontny HU, Antinore MJ, et al. Gadd45, a p53-responsive stress protein, modifies DNA accessibility on damaged chromatin. Mol Cell Biol 1999; 19: 1673-1685.
- Hwang BJ, Ford JM, Hanawalt PC, Chu G. Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc Natl Acad Sci U S A 1999; 96: 424-428.
Reference Link - Tanaka H, Arakawa H, Yamaguchi T, Shiraishi K, Fukuda S, Matsui K, et al. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature 2000; 404: 42-49.
Reference Link - Adimoolam S, Ford JM. p53 and DNA damage-inducible expression of the xeroderma pigmentosum group C gene. Proc Natl Acad Sci U S A 2002; 99: 12985-12990.
Reference Link - Sengupta S, Harris CC. p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol 2005; 6: 44-55.
Reference Link - Friedberg EC, Bond JP, Burns DK, Cheo DL, Greenblatt MS, Meira LB, et al. Defective nucleotide excision repair in xpc mutant mice and its association with cancer predisposition. Mutat Res 2000; 459: 99-108.
Reference Link - Hollander MC, Sheikh MS, Bulavin DV, Lundgren K, Augeri-Henmueller L, Shehee R, et al. Genomic instability in Gadd45a-deficient mice. Nat Genet 1999; 23: 176-184.
Reference Link - Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol 1987; 123: 241-250.
Reference Link - Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 1993; 75: 1027-1038.
Reference Link - Nystrom-Lahti M, Parsons R, Sistonen P, Pylkkanen L, Aaltonen LA, Leach FS, et al. Mismatch repair genes on chromosomes 2p and 3p account for a major share of hereditary nonpolyposis colorectal cancer families evaluable by linkage. Am J Hum Genet 1994; 55: 659-665.
- Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood 2003; 101: 822-826.
Reference Link - Auerbach AD, Allen RG. Leukemia and preleukemia in Fanconi anemia patients. A review of the literature and report of the International Fanconi Anemia Registry. Cancer Genet Cytogenet 1991; 51: 1-12.
Reference Link