Introduction
Multinodular goiter is the most common indication for surgery in endemic iodine-deficiency regions. Ever since Theodor Koher proposed surgery for goiter about a century ago there has been a debate about the best surgical resection for the disease. Nowadays the paradigm shift in thyroid surgery away from Kocher’s principles of nodal enucleation or subtotal resection to extended thyroidectomy involving at least hemithyroidectomy (total unilateral lobectomy) is a well-accepted approach for the treatment of multinodular goiter disease. The transition time in surgical practice, in our country from subtotal to total resection offered us a unique opportunity to compare the ultrastructure of the thyroid gland in primary and redo-operation in patients with nodular goiter. The structural particularities of the thyroid gland in various normal and pathological conditions [
Material and methods
For the purpose of this study we used operative material from patients from 20 to 69 years of age with euthyroid nodular goiter. The material derived from two groups –from patients with primary operations (50 patients) and patients with reoperation (50 patients). The tissues were prepared in accordance to the protocols for light microscopy examination and they were stained with Hematoxilin eosin and Masson technique [
Results
The results of our study showed that there are differences in the quantity and in the structure of the stroma of the thyroid gland before and after the operation. In the material from the first group we found that the stroma is represented by one or two rows of cells in the septum or in little group of cells in the space between the follicles. Examined under electron microscope the cells of the stroma had ultrastructural characteristics of fibroblasts - they had very well developed endoplasmic reticulum, Goldgy complex and a small number of vesicles and vacuoles. The proteoclican complexes were situated mainly in the territorial matrix (Fig. 1).
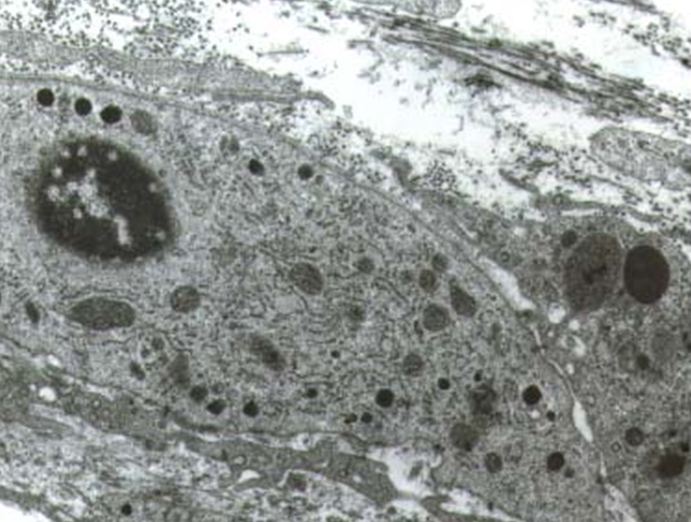
The examination of the material from the second group showed a significant thickening of the intrafollicular septum. We found an increase in the quantity of the cells and collagen bundles in the interfollicular space. The cells had smaller volume and less developed intracellular organs. The volume of the endoplasmic reticulum was decreased. The Goldgy complex was rarely found (Fig. 2). The formation of the collagen bundles is accompanied by the expansion of the cytoplasmic appendages of the fibroblasts. That is how they encircle an exact number of collagen filaments. There was a decrease in the number of proteoglycan complexes in the intracellular matrix. At the same time there was an increase in the capillary network of the stroma. In this group we found an increased number of fenestrated capillary. A great number of pinocitic vesicles in the luminal surface of the endothelial cells were detected. Pericytes were also observed.
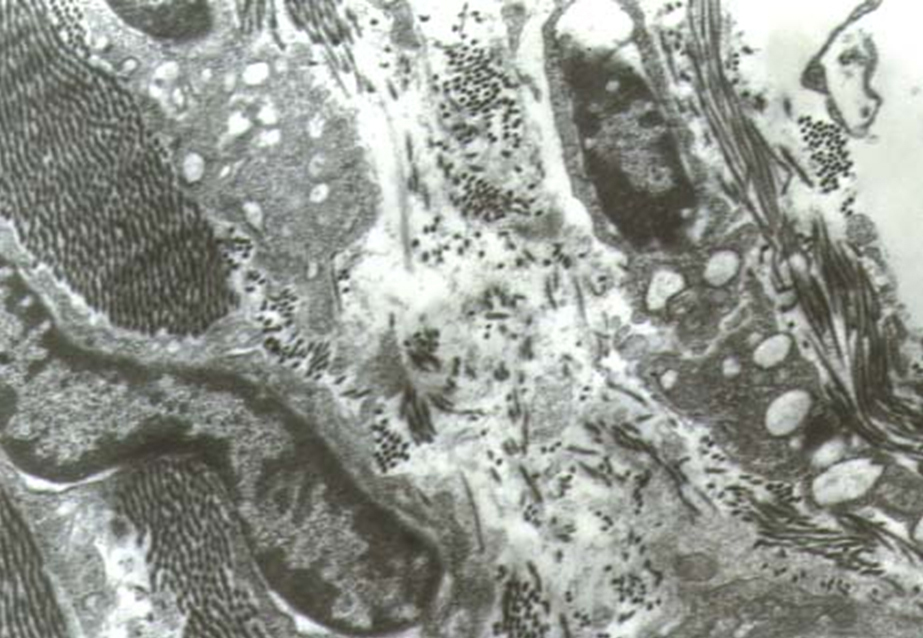
Discussion
Our studies showed that the connective tissue of the thyroid gland plays an important role in the formation of the tumors and in the processes of postoperative recovery of the gland. The changes in the connective tissue elements are more significant and occur earlier than in the epithelial cells [
Conclusions
The connective tissue of the thyroid gland reacts faster to the changes of the structure of the gland than the epithelial cells of the follicles. The main sign of the reaction is the intensification of the collagen production of the fibroblasts and the reduction of the proteoglycan complexes. These changes are pathological in nature and are the main cause for the decrease in thyroid function. This fact justifies a more extensive surgery.
References
- Brooks JR, Starnes HF, Brooks DC & Pelkey JN. Surgical therapy for thyroid carcinoma: a review of 1249 solitary thyroid nodules. Surgery 1988; 104: 940-946.
- Eberle F & Grun R. Multiple endocrine meoplasia, Type I. Ergeb Inn Med Kinderheilkd 1981; 46: 76-149
Reference Link - Feldman PS, Horvath E & Kovacs K. Ultrastructure of three Hürthle cell tumors of the thyroid. Cancer 1972; 30: 1279-1285.
Reference Link - Guo SS & Sawicki MP. Molecular and genetic mechanisms of tumorigenesis in multiple endocrine neoplasia type 1. Mol Endocrinol 2001; 15: 1653-1664.
Reference Link - Shaha AR, Loree TR & Shah JP. Prognostic factors and risk group analysis in follicular carcinoma of the thyroid. Surgery 1995; 118: 1131-1136.
Reference Link - Carcangiu ML, Bianchi S, Savino D, Voynick IM & Rosai J. Follicular Hürthle cell tumors of the thyroid gland. Cancer 1991; 68: 1944-1953.
Reference Link - Harness JK, Thompson NW, McLeod MK, Eckhauser FE & Lloyd RV. Follicular carcinoma of the thyroid gland: trends and treatment. Surgery 1984; 96: 972-980.
- Miller RH, Estrada R, Sneed WF & Mace ML. Hürthle cell tumors of the thyroid gland. Laringoscope 1983; 93: 884-888.
Reference Link - Prophet E, Mills B, Arrington J & Sobin L. Laboratory methods in histotechnology. American Registry of Pathology, 1992.
- Worthington BS & Enwonwu CO. Functional variations in the ultrastructure of the thyroid gland in malnourished infant monkeys. Am J Clin Nutr 1975; 28: 66-75.
- Tissell L, Hansson G & Jansson S. Reoperation in the treatment of asymptomatic metastasizing medullary thyroid carcinoma. Surgery 1986; 99: 60-66.
- Watson RG, Brennan MD, Goellner JR, van Heerden JA, McConahey WM & Taylor WF. Invasive Hürthle cell carcinoma of the thyroid: natural history and management. Mayo Clin Proc 1984; 59: 851-855.
Reference Link - Harrer P, Bröcker M, Zint A, Derwahl M, Barbera L & Zumtobel V. The clonality of nodules in recurrent goiters at second surgery. Langenbecks Arch Surg 1998; 383: 453-455.
Reference Link - Studer H, Gerber H, Zbaeren J & Peter HJ. Histomorphological and immunohistochemical evidence that human nodular goiters grow by episodic replication of multiple clusters of thyroid follicular cells. J Clin Endocrinol Metab 1992; 75: 1151-1158.
Reference Link - Böttcher Y, Eszlinger M, Tönjes A & Paschke R. The genetics of euthyroid familial goiter. Trends in Endocrinology & Metabolism 2005; 16: 314-319.
Reference Link - Brix TH & Hegedüs L. Genetic and environmental factors in the aetiology of simple goiter. Annals of Medicine 2000; 32: 153-156.