Introduction
Heterotrimeric G proteins, which consist of an a, b and g subunit , are involved in signalling pathways which regulate cell migration and chemotaxis both of which are prerequisites for metastasis formation [
Following the original findings of Siffert et al. [
Interestingly, a recent study has shown that both humans and transgenically engineered mice that carry an extra copy of GNB3 gene leads to obesity and increased weight and abdominal fat content in humans and mice respectively [
A previous cohort study in cancer patients with transitional cell bladder carcinoma reported that the GNB3 825T polymorphism influenced the biological behaviour of tumour disease [
Therefore understanding the effects of the splice variant Gβ3s protein subunit, in the Gβγ signalling pathway, may help us explain the complex disorders that 825T GNB3 individuals are predisposed to. However, due to the limited access to affected human tissues, currently only appropriate cellular models can be employed to decipher such signalling changes. B-lymphoblast cells, however, have previously been used as a convenient experimental cell system, that has led to the reproducible assessment and examination of complex molecular signaling networks [
Controversy exists in whether the presence of Gβ3s causes any signaling alterations unique to this truncated form leading to functional consequences, or if it is functionally unstable. For example, Virchow S et al reported that neutrophils of homozygous GNB3 (TT) genotype displayed enhanced migration kinetics as compared to neutrophils of heterozygous GNB3 825C/825T (CT) and homozygous GNB3 (CC) genotype [
Materials and Methods
Cell culture and treatments
Immortalised B-lymphoblast cell lines expressing 825CC (GM19116) Gβ3 and 825TT (GM18500) Gβ3s protein were obtained from Coriell Cell Repositories, USA. These cell lines were cultured in 10% FCS supplemented RPMI media (Invitrogen UK) in culture flasks. Simian COS-7 cells (ATCC CRL 1651) were maintained in DMEM (Invitrogen, UK) supplemented with 10% foetal bovine serum (FBS), 2 mM glutamine, 1 mM sodium pyruvate, 100 μg/ml streptomycin and 100U/ml penicillin in an atmosphere of 5% CO2. Cells were stimulated with Epidermal Growth Factor (EGF) or Transforming Growth Factor alpha (TGFα) or Vascular Endothelial Growth Factor (VEGF) (Invitrogen, UK). Treatments with inhibitors were performed with Verapamil, a calcium channel blocker at 20mg/ml [
Plasmids, transfections and stable clones
Both (pCDNA 3.0) His tagged GNB3 and GNB3s were constructed by PCR amplification of pREP4-GNB3 and pREP4-GNB3s cDNAs obtained from Dr. M.J Bullido (Universidad Autónoma de Madrid, Cantoblanco, Madrid, Spain) using primers carrying flanking restriction sites forward HindIII, 6x His tag (underlined) 5’AAGCTTATGCATCATCACCATCACCACACGGGGAGATGGAGCAACTG 3’ and reverse XhoI sites 5’ CTCGAGTCAGGTTCCAGATTTTAGG 3’. PCR amplified fragments were digested and sub-cloned into pCDNA3.0. The fidelity of the constructs has been checked and verified by sequencing using a commercial sequencing service (www.dnaseq.co.uk). COS-7 cells were transfected using electroporation. When they reached confluence at 2 x 107, cells were washed in PBS and resuspended in 400μl of the Optimem transfection media (Invitogen UK), and were electroporated using settings at 400V, 350μF, using Easyject EquiBio (Kent UK). 10μg DNA was used per transfection. Subsequently, stable clones expressing 6xHis-tagged Gβ3 (Gβ3-COS) and Gβ3s (Gβ3s-COS) were generated by selection with G418 (Invitrogen UK) at 800 μg/ml, and subsequently maintained at 400 μg/ml. The culture was placed back in the incubator until individual colonies were visible. Individual colonies were expanded, and the level of 6x Histidine-tagged Gβ3 and Gβ3s protein expression was determined by western blot analysis using anti-His antibody mentioned in Table 1.
Antibodies, immune-blotting and data presentation
Expression of Gβ3 and Gβ3s protein was detected using Anti- rabbit GNB3, anti-sera on B lymphoblast cells at a relevant dilution (1:2000) in blocking reagent. The antibodies mentioned in table 1 are used to detect the expression of phosphorylated and total proteins levels in B lymphoblast cell lysates. Rho A, Rho B, Rho C, Rac 1-2-3 and CDC42 total and phosphorylated expression were determined using Rho GTPase antibody sampler kit (Cell signalling, UK). Briefly, by centrifuging cells at 1500g for 5min, the obtained pellet was washed twice with ice cold PBS and centrifuged again at 1500g for 5 min. 1 ml of RIPA buffer (Pierce Biotechnology UK) was added to 40 mg of wet cell pellet, mixed well and then sonicated for two cycles of 5 seconds at 50% pulse. The final mixture was shaken gently on ice for 15 min and the protein supernatant was obtained by centrifuging the cells at 14000g for 15 min. Subsequently all protein extract samples were quantified with the use of a spectrophotometer, with BSA as a standard. 50mg cell lysates were mixed with sample buffer and equal concentrations were loaded on 10–12% SDS–polyacrylamide gels. After electrophoresis, the proteins were transferred to nitrocellulose membranes (Bio-Rad Hybond ECL). Further blotting was carried out using a commercially available kit (QDOT® 625 Streptavidin conjugate kit, Invitrogen, UK). Non-specific reactivity was blocked by incubation with a blocking reagent supplied in the kit. Membranes were further processed by incubating them with relevant primary antibodies described in Table 1, washed and further incubated for an hour (hr) with Biotin labelled secondary antibodies. Finally, bands were visualized with the enhanced streptavidin substrate, washed and visualised under the UVDOC imaging system (Alpha Innotech). For internal control, the levels of β-actin were examined on the same blot by incubating the blots with stripping solution for 1 hr (20% SDS, 67.5µM Tris-HCl of a pH 6.7, 100µm 2- Marcaptoethanol) and reprobing with anti-β-actin (Sigma-Aldrich, UK) and anti-mouse IgG secondary antibody (Sigma-Aldrich, UK). Densitometry analyses on the immunoblotted images were performed by Gelpro software (Media Cybernetics, USA). Statistical analysis was performed using Graph Pad Prism (USA) and displayed as mean ± S.E. The significance (p value) of differences of pooled results was estimated by ‘t’ tests, and were defined as *p< 0.05.
FITC conjugated cholera toxin B (CTB) uptake, Immunocytochemistry (ICC), imaging and co-localisation
Stable expressing His-Gβ3 and His-Gβ3s COS cells were grown on standard glass coverslips coated with polylysine (Sigma-Aldrich, UK). They were placed in petri dishes containing Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, UK) supplemented with 10% fetal bovine serum and allowed to grow for 18-24hrs. Following relevant treatments, cells were fixed in 3% paraformaldehyde in a standard PBS at room temperature for 30 minutes (min). The cells were then gently washed twice with 1 ml of PBS, and blocked using 1% goat serum and 1% bovine serum albumin in PBS containing 0.05% Triton X-100, for 30 min. They were then incubated with a primary antibody diluted in blocking solution for 30 min, washed three times with the 0.3% Triton X-100/PBS for 5 min, and then incubated with the relevant Alexa fluor 488 and Alexa Flour 568 conjugated goat anti-rabbit secondary antibodies (Invitrogen UK) for 30 mins. Fluorescent Alexa Fluor 568 phalloidin (Invitrogen UK) was used to visualize actin structures. For nuclear staining, cells were mounted with DAPI containing Vectashield solution (Vector Laboratories UK). Cells showing actin filament bundles across cell bodies were counted as stress fiber positive, cells showing mainly cortically distributed actin filaments were counted as stress fiber negative. Mounted cells were imaged under a Leica DMiRe2 electronic controlled microscope, under relevant excitation and emission filters depending on the fluorotype. An iXonEM +897 EMCCD camera (ANDOR technologies ltd, USA) was used to capture images and visualisation was performed using multi-dimensional microscopy software Andor Module iQ Core. Colocalisation assays were performed and determined with software integral features supplied by Andor IQ core software features.
Migration assay
Migration assay was performed using the 48-well Boyden chambers (8 μm pores, BD Biosciences). The cells were plated at a density of 1 × 106 cells/100 μl serum-free media containing 10% BSA which was used as a positive control. This was placed in the bottom wells of a Boyden chamber. A medium containing lymphoblast cells, starved for 2 hr in serum free media, was added to the top chamber. For the inhibition assays, cells were starved for 2 hr and pre-incubated with inhibitors for 30 min. Inhibitors were also added to the cell suspension placed on the top chamber wells, and also to the chemoattractant added to the bottom chamber wells, in order to be present during the migration time. After 6 hr incubation the cells were stained using the Diff-Quick kit (Dade Behring). Pictures were taken with a light microscope and migrated cells were counted with Gelpro Software (Media cybernetics, USA) using 10 randomly chosen fields.
Calcium influx assay
Lymphoblast cells at 125000 cells / well density were plated in a 96 well plate and incubated for 16hrs in RPMI media (Invitrogen UK). 16 hrs later RPMI media without serum was added prior to stimulation with EGF. Upon EGF stimulation at relevant time points, cytosolic calcium influx assay was carried out using Molecular Probes’ Fluo-4 NW (No-Wash) Calcium Assay Kit (Invitrogen UK) following the kit instructions. Calcium response was detected using the Modulus™ Microplate Multimode Reader plate reader using instrument settings appropriate for excitation at 494 nm and emission at 516 nm. Functional parameters were determined with Graph Pad PRISM software using non-linear regression applied to a sigmoidal dose response model.
cAMP assay
cAMP assays were carried out as mentioned in Tummala et al 2011 [
Scratch wound assays
Stable COS-7 clones expressing His-Gβ3 and His-Gβ3s subunits were plated onto poly-d-lysine-coated coverslips (10 μg/ml). These were grown to 100% confluence, and serum starved for 12hr. Wounds were created by scraping the monolayer of cells with a sterile pipette tip and culture media was added with or without inhibitors. The images of wounds were captured at 5x magnification with a Leica DMiRe2 electronic controlled microscope immediately after wounding and again 24 h later. Images were collected under bright field using an iXonEM +897 EMCCD camera (ANDOR technologies ltd, USA) and visualised using multi-dimensional microscopy software, Andor Module iQ Core. The images were subjected to the WIMASIS image analysis application that is available for use on the Wimasis my Wim platform. The data is presented as cell covered area at noted time points and estimates the effects of inhibitors on cell migration.
Results
Expression and characterisation of Gβ3s subunit
EBV transformed Gβ3 expressing lymphoblastoid cells (CC) and Gβ3s/Gβ3 expressing lymphoblastoid cells (TT) were grown in relevant media, harvested and lysed to obtain protein lysates. Following processing of the lysates for immunoblotting, SDS-PAGE was performed and the blot was probed with a custom raised Gβ3 specific antibody [
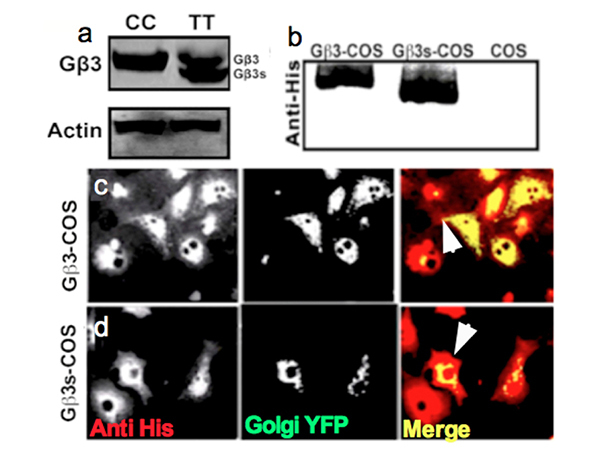
It has been known that in culture cells, Gβ and Gγ subunits interact to rapidly form the irreversible Gβγ dimer following synthesis [
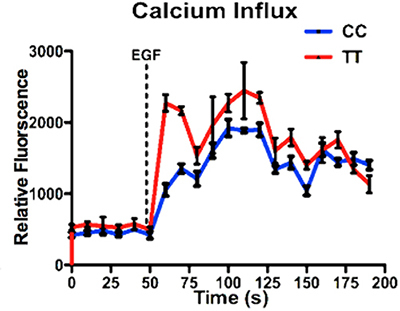
Epidermal Growth Factor (EGF)-induced Ca2+ ion influx was enhanced in the presence of Gβ3s
Intracellular Ca+ ions are known to be modulated following G-Protein Coupled Receptors (GPCRs) stimulation via Gβγ [
Gβ3s induces ERK activity and activates mTOR pathway but not AKT1
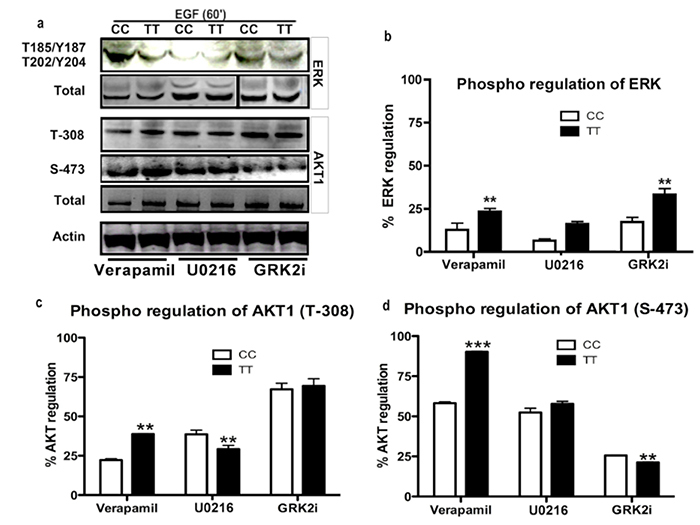
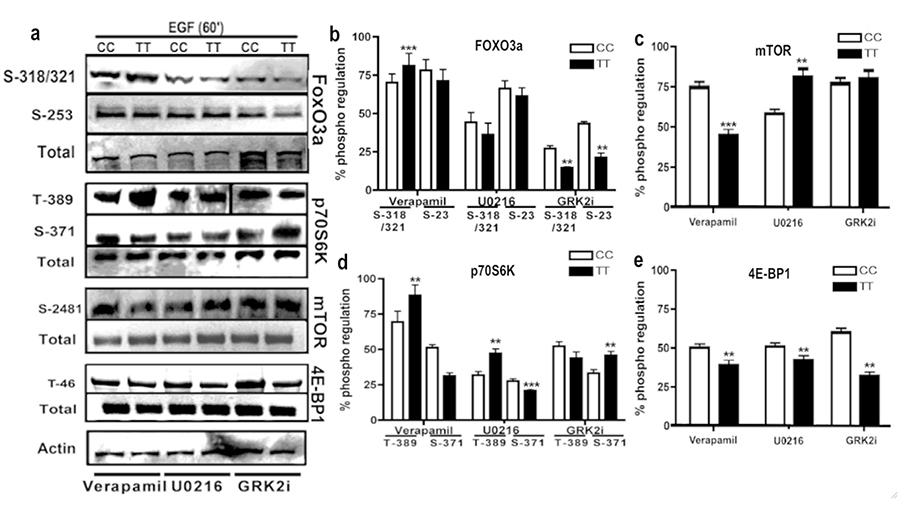
MAPK cascades are evolutionarily conserved in regulating cell signal transduction by connecting cell-surface receptors to critical regulatory targets within cells. These pathways are essential in controlling cell survival, proliferation and apoptosis. Since both Receptor tyrosine kinases as well as GPCRs signal mainly via PI3K and MAPK pathways, we wanted to determine whether the presence of Gβ3s will have any impact on the key effector proteins of these pathways. We found that both TT lymphoblastoid and Gβ3s-COS cells had significantly higher basal levels of ERK phosphorylation (figure 3 a). Probing for the presence of phosphorylated AKT1 on the contrary revealed a reduction in basal Threonine-308 (T-308) and Serine-476 (S-473) AKT1 phosphorylation in both TT lymphoblastoid and Gβ3s-COS cells as compared to their normal counterparts (figure 3a and 3g). Interestingly, both TT lymphoblastoids and Gβ3s-COS also showed enhanced mTOR phosphorylation at Serine 2481 (S-2481) despite the reduction in AKT1 phosphorylation (figure 3b). This prompted us to look at downstream phospho induction of mTOR substrates. As shown in figure 3b, enhanced mTOR phosphorylation was also accompanied by induction of its substrates p70S6K [phospho Threonine 389 (T-389) and phospho Serine 371 (S-371)] and 4E-BP1 [phospho Threonine 46 (T-46)] indicating hyperactivation of mTOR pathway in Gβ3s containing cell lines.
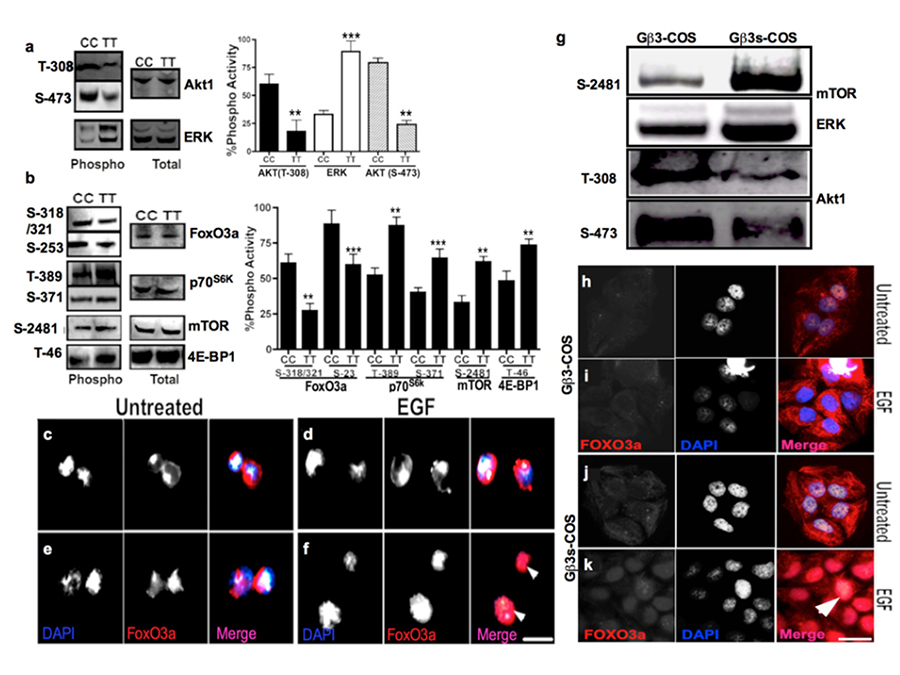
Previous studies have reported that agonists of GPCRs potentiate the mitogenic effect by promoting a sustained late phase activation of PI3K and p70S6K via a pathway dependent on the Gβγ subunits of heterotrimeric G proteins [
Gβ3s promotes nuclear accumulation of Foxo3a suggesting an increase in its transcriptional activity
AKT activation leads to phosphorylation of Foxo3a that results in its exclusion from nucleus and sequestration in cytoplasm, thereby inhibiting its transcriptional function [
Immunostaining of subcellular Foxo3a revealed that Gβ3s induced reduction of phospho Foxo3a also led to an increase in its nuclear accumulation in constitutive state (figure 3 e & j) as well as following stimulation with EGF (figure 3 f & k) in our cell line models. This particular nuclear staining appears to be absent in CC lymphoblast (figure 3c-d) and Gβ3-COS cells (figure 3h-i) expressing normal Gβ3 subunit. These figures suggest that loss of AKT phosphorylation in the presence of Gβ3s may lead to Foxo3a mediated transcriptional induction and hence present an altered signaling profile as compared to cells harboring the normal copy of the gene.
Presence of Gβ3s variant causes downregulation of Cyclic AMP and increased phosphorylation of Caveolin 1a in response to EGF stimulation
Gβγ signaling has been shown to play a role in modulating intracellular levels of second messenger, cyclic AMP (c-AMP) and its function has been implicated in both c-AMP increase [
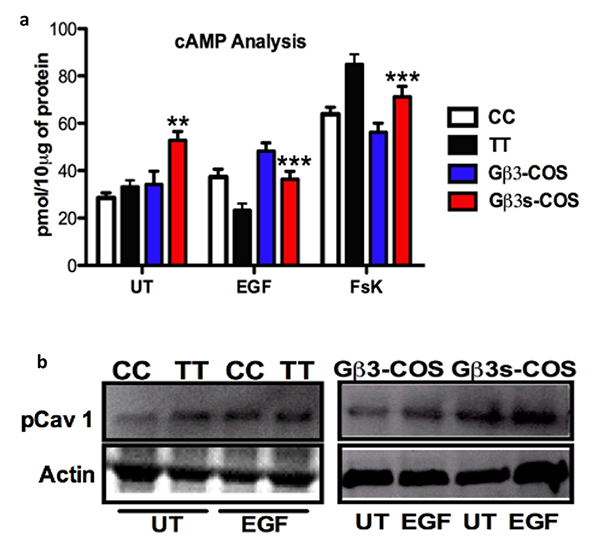
Altered cAMP regulation in TT lymphoblastoid cell line can implicate differences in various signaling processes including differential regulation of the microenvironment by recruitment of scaffolding molecules that include caveolae [
These previous reports as well as insights from current investigation led us to propose that the phosphorylated levels of pCav1 might be altered in Gβ3s containing cells as compared to their normal counterparts. Indeed we found that both at basal levels and upon EGF stimulation, the levels of phosphorylated Cav1 at tyrosine 14 (Y-14) residue in both Gβ3s-COS and TT lymphoblast cells showed an induction in comparison to Gβ3-COS and CC lymphoblast cells respectively (figure 4b) thus confirming our hypothesis. These studies also suggest that Gβ3s signaling may be involved in cytoskeletal remodeling and maintenance of cell shape, features that regulate cell migration and metastasis, by modulating c-AMP levels that result in changes in Caveolae microdomains.
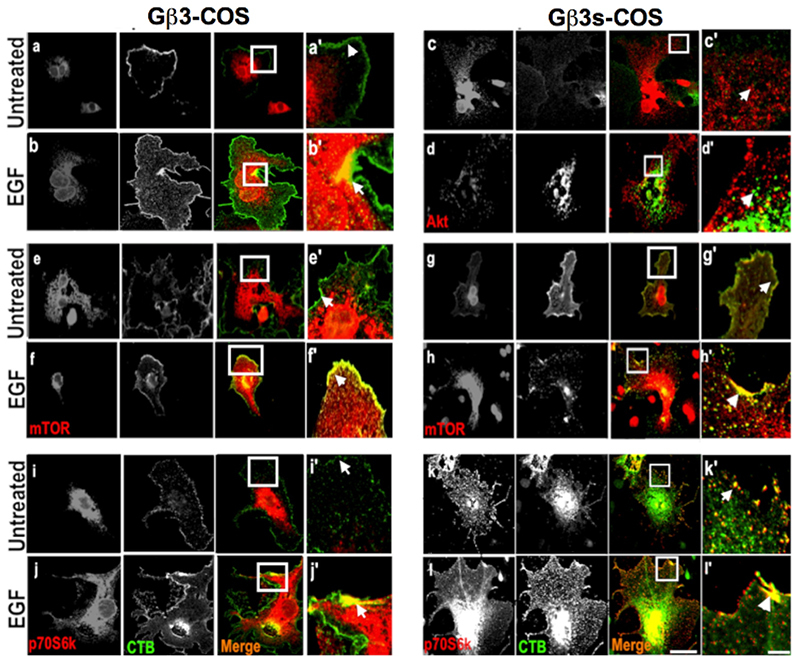
AKT1 localisation towards caveolar membrane free domains is reduced in the presence of Gβ3s subunit
Following the previous finding that Cav1 phosphorylation was enhanced in Gβ3s containing cells, we next wanted to determine at a cellular level whether there is an accompanying altered localisation of substrates of PI3K pathway at caveolae. This was done owing to the fact that we had found altered phosphorylated levels of PI3K substrates that can lead to a substantial change in their sub-cellular compartmentalisation.
Monitoring the cholera toxin B (CTB) subunit internalisation in mammalian cells has previously been reported as a marker for uptake via the caveolae-dependent pathways [
Gβ3s subunit localises in and enhances actin stress fibers formation
The cell cytoskeleton is a network of structural fibers found within the cytoplasm of a cell. It is responsible for cell movement and shape stability. Actin microfilaments are one of the three major types of fibers that form the cell cytoskeleton. Through their association with motor proteins such as myosin, actin microfilaments carry out cellular movements [
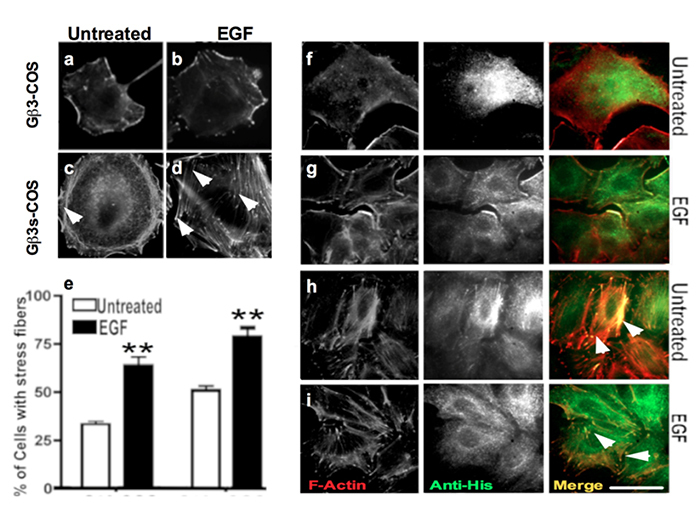
Actin reorganisation and modeling, which is a key element in cell migration, has previously been shown to be in part dependent on PI3K and Ras pathway, independent of GPCRs, where PI3K was found to localise at sites of F-actin projection [
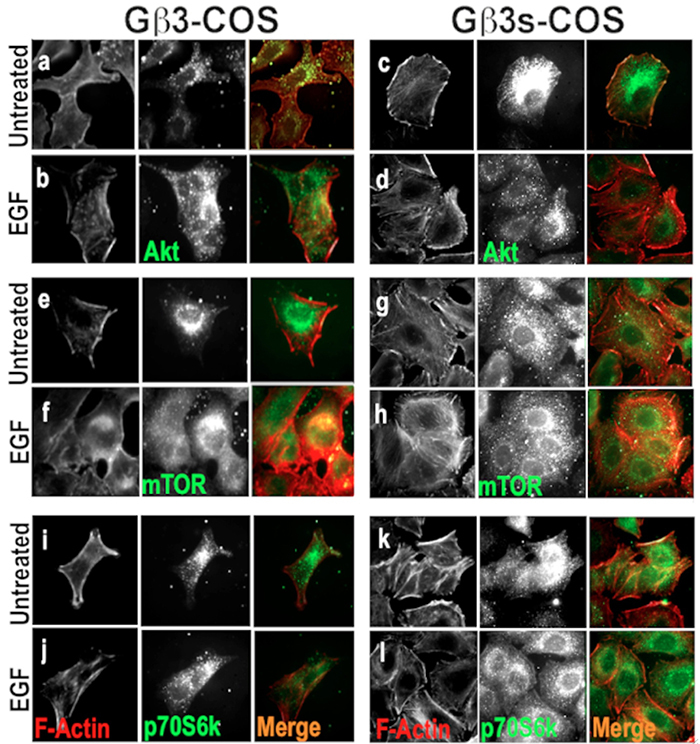
The altered localisation of total AKT in Gβ3s-COS cells at both basal and EGF stimulated conditions indicate that the reduced AKT S-473 and T-308 phosphorylation in TT lymphoblast and Gβ3s-COS cells (figure 2a & 3g), impaired its co-localisation with actin filament assembly.
Presence of Gβ3s variant enhances cell migration and scratch wound healing
A number of findings made in our investigation uncovered signaling alteration specific to the presence of Gβ3s not only in PI3K and MAPK pathway substrates, but also in second messengers and overall cytoskeletal remodeling. Cav-1 expression and phosphorylation is known to be associated with enhanced cell migration especially in fibroblasts, where caveolin-1 expression and phosphorylation at Y-14 residue are proven to be essential to promote migration [
To answer this, we studied cell migration by performing boyden chamber based migration assays using different migratory and Ca2+ stimulating ligands on Gβ3s/Gβ3 expressing TT lymphoblastoids and only Gβ3 expressing CC lymphoblastoid cells. We found that TT lymphoblastoid cells under control (BSA stimulated) conditions had a significantly larger number of migrating cells than CC cells (figure 8a). Moreover, following ligand stimulation by EGF, Vascular Endothelial Growth Factor (VEGF) and Transforming Growth Factor α (TGFα), the number of migrating cells increased for both CC and TT cell types (figure 8a). However, upon ligand stimulation, the latter cell type showed a significantly greater number of migratory cells than the former, especially for EGF treatment, which led to almost twice the number of motile TT cells as compared to CC (figure 8a). The migratory difference following EGF stimulation is also displayed as a % change from the basal rate, normalized to 100% for both CC and TT cell types (figure 8b). According to this figure, following stimulation with ligand, the % of migrated cells doubled in TT cells, as compared to CC cells where only a modest increase was observed.
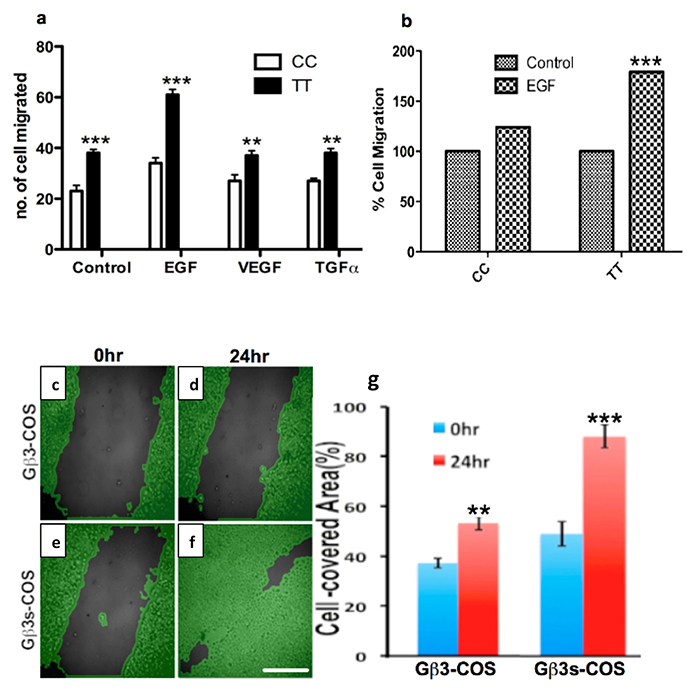
In a similar experiment, scratch wound healing assays on stable COS7 clones of Gβ3 and Gβ3s was performed. Scratch wound healing assay revealed enhanced kinetics of wound healing of Gβ3s-COS cells (figure 8f & g) as compared to Gβ3-COS cells (figure 8d & e) 24hrs post scratch and stimulation with EGF. The enhanced surface area covered by Gβ3s-COS when compared to Gβ3-COS post scratching reveals that Gβ3s subunit expression (figure 8f) induced enhanced migration kinetics, but not cell proliferation (figure 1a & b).
Taken together, these findings suggest that lower comparative activation of AKT1, but enhanced mTOR and downstream substrate phosphorylation suggest that a second regulatory pathway responds to growth factor stimulation only in the presence of the Gβ3s subunit to promote enhanced migration.
Gβ3s-induced cell migration is dependent upon Ca2+ influx-regulated ERK but independent of AKT1 and mTOR
Gβγ effector signalling is primarily regulated through Ca2+ ion flux, which in turn activates various signaling pathways that control cell migration [
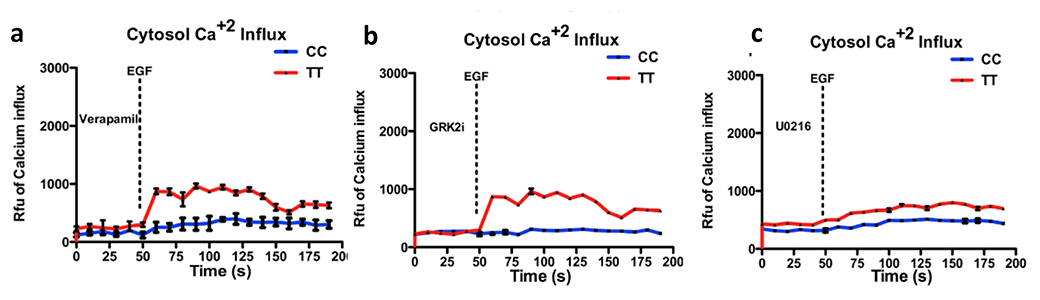
First of all, in order to characterize the role of Ca2+ ion flux in Gβ3s induced migration, we blocked the calcium channels using verapamil, an L-type calcium channel blocker. We saw inhibition of EGF stimulated intracellular Ca2+ ion influx following verapamil treatment in both TT and CC cells. However, this inhibition was to a lesser extent in TT lymphoblasts as compared to the CC counterparts (figure 9a). Interestingly, verapamil treatment led to increased AKT1 phosphorylation at both T-308 and S-473 residues in CC and TT cells (figure 10a & c) as compared to untreated cells (figure 3b). As expected, this AKT1 activation also led to a corresponding increase in Foxo3a phosphorylation in EGF stimulated cells following Verapamil treatment (figure 11a). To confirm the role of Ca2+ ion influx in regulating ERK activation, we blotted for total and phospho ERK and found that upon Ca2+ influx inhibition, phospho ERK levels dropped and the degree of reduction of ERK activity corresponded well with the degree of Ca2+ ion inhibition achieved with verapamil in the CC and TT cell line models (10 a & b). This confirmed the earlier assumption that EGF stimulated ERK induction is as a result of enhanced cytosolic Ca2+ ion influx in Gβ3s containing cells. Moreover, while the phosphorylated levels of mTOR and 4E-BP1 showed a decrease upon inhibition of Ca2+ influx (figure 11c & e), there was an overall increase in p70S6K T-389 phosphorylation (figure 11d) in TT cells as compared to the CC counterparts.
In Rat-1 fibroblasts, phosphorylation of 4E-BP1 was shown to occur via a pathway that was Ca2+ and calmodulin dependent [
We next studied the effects of MEK inhibition on these signaling components. Targeting MEK in the Ras/Raf/MEK/ERK pathway is advantageous as it is a convergence point for upstream signaling pathways controlled by AKT and ERK. ERK is known to be activated by MEK in a calcium sensitive pathway also involving modulation by cAMP via PKA. We found maximum inhibition of EGF induced cytosolic Ca2+ influx upon treatment with U0216, a MEK inhibitor (figure 9c). This correlates well with inhibition of ERK phosphorylation as expected (figure 10a).
Treatment of U0216 resulted in modest induction of AKT phosphorylation at both T-308 and S-473 residues in CC and TT lymphoblasts (figure 10a & d) when compared to untreated cells (figure 3b). A significant decrease in phospho 4E-BP1 threonine-46 residue was observed (figure 11a & e) upon MEK inhibition even though there was activation of mTOR and p70S6K (figure 11a, c & d). This suggests that the mTOR pathway activation via MEK inhibition might not involve a protein translation function. Previous studies reported the participation of Raf-1/MEK/ERK in crosstalk with 4E-BP1, leading to increased protein synthesis, cell growth, and kidney tumor formation (54). The drop in 4E-BP1 phosphorylation observed here or alternatively its activation (figure 3b) corresponded well with ERK activity rather than mTOR>p70S6k activation in both CC and TT lymphoblast cells (figures 10 & 11).
Next, in order to directly target upstream signaling emerging from G proteins, we used a GRK2 polypeptide (GRK2i), which acts as a cellular Gβγ antagonist by inhibiting binding to Gβγ dimer and sequester its signaling activity [
We finally studied the effects of the above treatments on cell migration of TT and CC cells, kinetics of scratch wound healing in Gβ3-COS and Gβ3s-COS cells and actin stress fiber formation. We found that inhibition of cytosolic Ca2+ influx following verapamil treatment inhibited EGF induced migration of TT lymphoblast cells down to their untreated levels (figure 12a). This implied the involvement of Ca2+ dependent activation of ERK in the enhanced migration of Gβ3s cells as well as an AKT independent phenomenon. This further suggests that Gβγ signaling induces hyper Ca2+ influx, which primarily contributes to the activation of ERK and mTOR activity, leading to enhanced migration only in the presence of a Gβ3s subunit but not in its absence. In a similar experiment, we found that the kinetics of wound healing in Gβ3s-COS were also significantly reduced upon verapamil treatment (compare figure 2e & f with figure 13g & h) also probably explaining the absence of EGF induced actin stress fiber formation following such treatments (compare figures 6d & 13x).
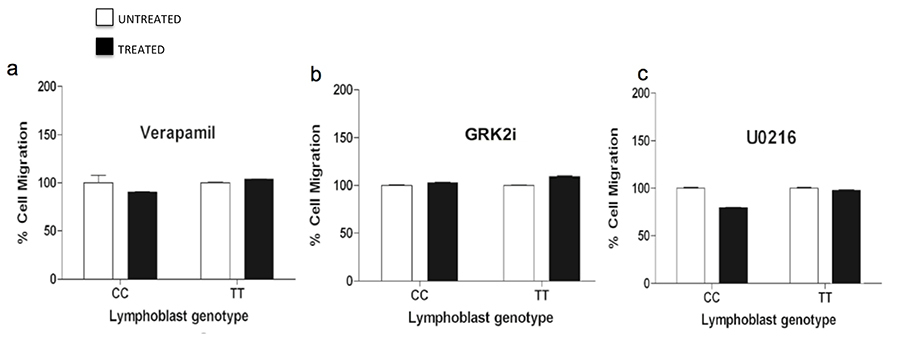
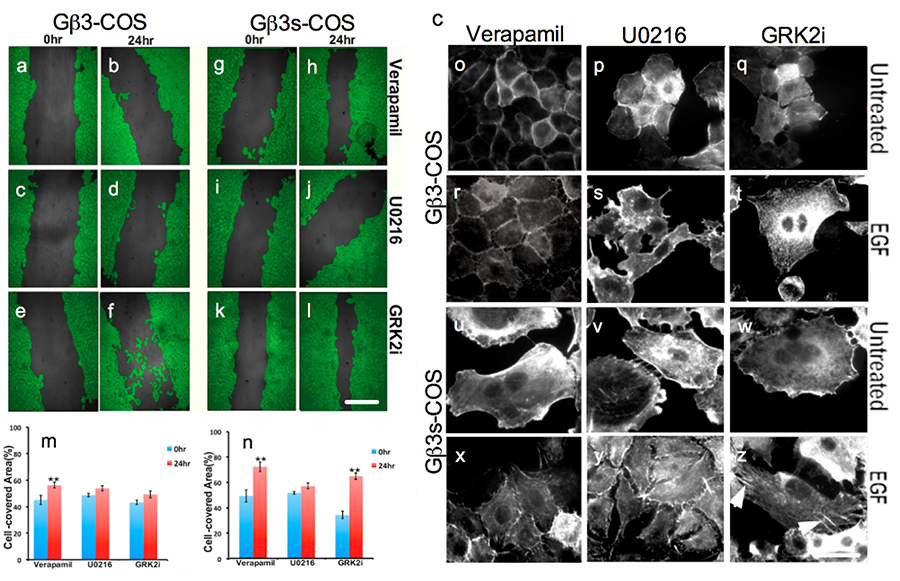
Hence, the elevation of AKT T-308 residue phosphorylation upon Verapamil treatment was not sufficient to potentiate migration kinetics in both the CC and TT lymphoblast cells (figure 12a) and COS7 stable clones (figure 13 a, b & g, h & 12 m, n). This implied that at signal effector level, the involvement of ERK was critical for the enhanced Gβ3s migration. Indeed, when the MEK inhibitor, U0216 was employed, which as expected, inhibited phospho ERK levels, not only abrogated cytosolic Ca2+ levels (figure 9c), but also resulted in complete inhibition of enhanced cell migration of TT cells (figure 12c), closer to 0% closure of the scratched surface after 24hr of healing time (figure 13i & j) and absence of any stress fiber formation (figure 13y). Treatment with GRK2i could not completely inhibit the EGF induced enhanced migration of Gβ3s cells (figure 12b) and there was some degree of scratch wound healing (figure 13 k & l). Failure of GRK2 inhibition to exert complete disruption of migration could be because it could not completely prevent EGF induced Ca2+ influx (figure 9b), or phospho ERK induction (figure 10b).
Altogether, these results indicate hyperactivation of a signaling pathway that is unique to the presence Gβ3s, which provide for a mechanism of enhanced migratory phenotype exhibited only by cells containing the Gβ3s variant and not normal Gβ3 protein. According to this mechanism, EGF stimulation leads to induction of supranormal levels of cytosolic Ca2+ that activate and cross-talk with MAPK pathway resulting in hyperphosphorylated ERK. The low levels of cAMP and phospho AKT in Gβ3s containing cells also result in activation of Foxo3a transcription factor and phosphorylation of Cav1 caveolae protein that contribute in cytoskeletal remodeling, formation of actin stress fibers and enhanced migration upon growth factor stimulation.
Gβ3s subunit signaling leads to Rho, Rac, and cdc-42 GTPases activation
Members of the Rho family of small guanosine triphosphatases have emerged as key regulators of the actin cytoskeleton, through their interaction with multiple target proteins [
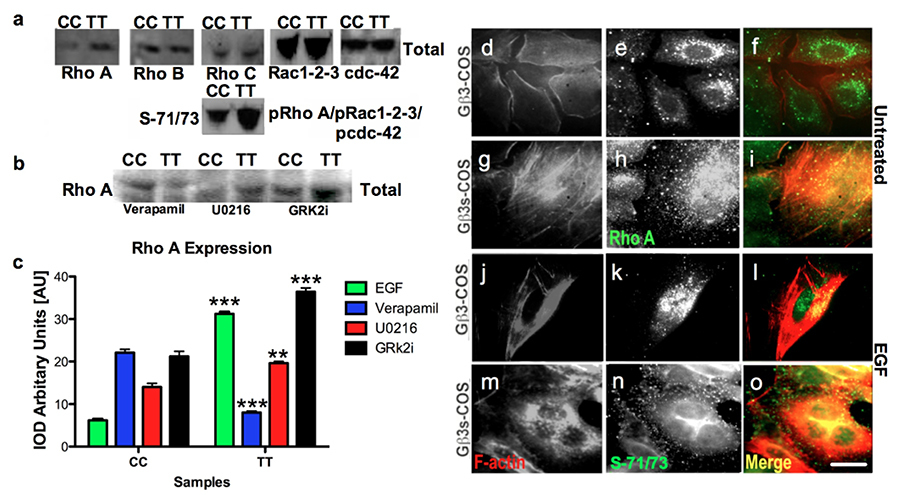
To our surprise, in Gβ3s subunit expressing TT lymphoblasts, we observed enhanced Rho A expression and also increase in phosphorylation activity of RhoA and Rac1-2-3/CDC-42 at S-71/73 respectively (figure 14a). TT lymphoblast cells showed decreased expression of Rho A when treated with verapamil in comparison to treatments with other inhibitor (figure 14b). Hence, Rho A expression showed correlation with cytosolic Ca2+ ions (figure 2). The enhanced RhoA expression and activity in Gβ3s-COS cells appeared to localise in actin stress fibers at both basal and EGF stimulated conditions (figure 14g, i & m & o) in comparison to the RhoA expression in Gβ3-COS cells (figure 14d, f & j & l).
Discussion
This study reports interesting findings of Gβ3s subunit signaling in regulating and stimulating distinct molecular dynamics affecting AKT and ERK activation. This occurs through the induction of cytosolic Ca2+ influx activating ERK, mTOR and p70S6K leading to reorganisation of actin cytoskeleton to enhance cell migration.
We observed hyper induction of cytosolic Ca2+ ions in response to EGF stimulation only in Gβ3s subunit expressing cells (figure 2). The rise in cytosolic calcium is likely to activate classical isoforms of protein kinase C (PKC), which moves from the cytosol to the membrane, where it is further activated by DAG [
G proteins regulate AKT1 by two distinct and potentially opposing mechanisms. These are by activation through Gβγ dimers in a PI3k dependent fashion and inhibition mediated by Gaq. Hence AKT1 is considered as a novel point of convergence between disparate signaling pathways. AKT1 serves as a multifaceted intermediary signaling to the diversified downstream biological effectors and as such has received much attention for the versatile roles it plays in various biological processes. Therefore the activation of AKT substrates such as mTOR (figure 3b) in the absence of AKT activation itself is a consequence of cross talk probably due to high ERK phosphorylation (figure 3a) resulting from the signaling unique to the presence of the Gβ3s subunit. Reduction of AKT1 T-308 phosphorylation in TT lymphoblast cells (figure 3a) might suggest that in the presence of Gβ3s, the targets of AKT1 phosphorylation (T-308) such as GSK3α/β, Foxo transcription factors, Bad, p21Cip1, and p27Kip1 will be affected. Enhanced phosphorylation of mTOR, p70S6K and ERK in the absence of AKT1 activation in TT lymphoblast cells suggests that the effectors of AKT1 might be redirected for control through ERK to compensate for the lack of AKT1 signaling. Phosphorylation of PI3K results in the conversion of PIP2 to PIP3. PIP3-triggered translocation of AKT1 from the cytoplasm to the plasma membrane is a prerequisite for its phosphorylation and activation [
Recent studies have also shown that the AKT2 isoform is an enhancer of cell migration and invasion in vitro and in vivo, and the related AKT1 isoform may function as an inhibitor of these metastatic phenotypes [
The observed signaling alterations unique to the presence of Gβ3s could exhibit a distinctive translational control. Firstly, Foxo3a is a key regulator of ubiquitin mediated proteolysis [
In adult cardiomyocytes, tissue plasminogen activator was shown to activate p70S6K in a non-AKT dependent manner through c-Raf/ERK1/2 pathway [
Interestingly, previous reports have shown that Gβγ signaling regulates Ca2+ fluxes and the exchange protein activator of cAMP (Epac) to induce cell migration in melanoma cell lines [
Y-14 phosphorylation of Cav1 is known to be necessary for binding of Cav1 to intermediate filaments, which in turn is required for anterior polarisation at focal adhesion sites for directional transmigration in cells [
Gβ3s is primarily implicated in predisposition to hypertension and various cardiac diseases [
Acknowledgements
HT, HSK and NZ would like to acknowledge the support given by the Northwood Charitable Trust. MRI is supported by SORSAS award and work in IRE and SJ laboratory is supported by Tattersall Trust and an anonymous trust.
References
- Entschladen F, Zänker KS, Powe DG. Heterotrimeric G protein signaling in cancer cells with regard to metastasis formation. Cell Cycle 2011; 10(7): 1086-91.
Reference Link - Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR,Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev 2003; 24(6): 765-81.
Reference Link - Guzmán-Hernández ML, Vázquez-Macías A, Carretero-Ortega J, Hernández-García R, García-Regalado A, Hernández-Negrete I et al. Differential inhibitor of Gbetagamma signaling to AKT and ERK derived from phosducin-like protein: effect on sphingosine 1-phosphate-induced endothelial cell migration and in vitro angiogenesis. J Biol Chem 2009; 284(27): 18334-46.
Reference Link - Tang X, Sun Z, Runne C, Madsen J, Domann F, Henry M et al. A critical role of Gbetagamma in tumorigenesis and metastasis of breast cancer. J Biol Chem 2011; 286(15): 13244-54.
Reference Link - Kirui JK, Xie Y, Wolff DW, Jiang H, Abel PW, Tu Y. Gbetagamma signaling promotes breast cancer cell migration and invasion. J Pharmacol Exp Ther 2010; 333(2): 393-403.
Reference Link - Baljinnyam E, Umemura M, De Lorenzo MS, Xie LH, Nowycky M, Iwatsubo M et al. Gβγ subunits inhibit Epac-induced melanoma cell migration. BMC Cancer 2011; 11(256): 1471-2407
- Castellano E, Downward J. RAS Interaction with PI3K: More Than Just Another Effector Pathway. Genes Cancer 2011; 2(3): 261-74.
Reference Link - Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005; 24(50): 7455-64.
Reference Link - Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, Erbel R., et al. Association of a human G-protein β3 subunit variant with hypertension. Nat Genet 1998; 18(1): 45–48.
Reference Link - Tummala H, Ali M, Getty P, Hocking PM, Burt DW, Inglehearn CF, Lester DH. Mutation in the guanine nucleotide-binding protein beta-3 causes retinal degeneration and embryonic mortality in chickens. Invest Ophthalmol Vis Sci 2006; 47(11): 4714-8.
Reference Link - Tummala H, Fleming S, Hocking PM, Wehner D, Naseem Z, Ali M, et al. The D153del mutation in GNB3 gene causes tissue specific signalling patterns and an abnormal renal morphology in Rge chickens. PLoS One 2011; 6(8): e21156.
Reference Link - Virchow S, Ansorge N, Rosskopf D, Rübben H, Siffert W. The G protein beta3 subunit splice variant Gbeta3-s causes enhanced chemotaxis of human neutrophils in response to interleukin-8. Naunyn Schmiedebergs Arch Pharmacol 1999; 360(1): 27-32.
Reference Link - Tummala H, Khalil HS, D Ascanio I, Wehner D, Lester DH, Babraj John et al. Human 825C>T polymorphism in GNB3 gene promotes enhanced cell migration by inducing cytosolic calcium influx and hyper phosphorylation of ERK regulated mTOR pathway. FEBS J 2012; 279(1) p. 141.
- Goldlust IS, Hermetz KE, Catalano LM, Barfield RT, Cozad R, Wynn G, et al. Mouse model implicates GNB3 duplication in a childhood obesity syndrome. Proc Natl Acad Sci U S A 2013; 110(37): 14990-4.
Reference Link - Dobrev D, Wettwer E, Himmel HM, Kortner A, Kuhlisch E, Schüler S, Siffert W,Ravens U. G-Protein beta(3)-subunit 825T allele is associated with enhanced human atrial inward rectifier potassium currents. Circulation 2000; 102(6):692-7
Reference Link - Lindemann M, Virchow S, Ramann F, Barsegian V, Kreuzfelder E, Siffert W,Müller N, Grosse-Wilde H. The G protein beta3 subunit 825T allele is a genetic marker for enhanced T cell response. FEBS Lett 2001;495(1-2):82-6.
Reference Link - Siffert W, Rosskopf D, Moritz A, Wieland T, Kaldenberg-Stasch S, Kettler N, Hartung K, Beckmann S, Jakobs KH. Enhanced G protein activation in immortalized lymphoblasts from patients with essential hypertension. J Clin Invest 1995;96(2):759-66.
Reference Link - Weinstein LS, Chen M, Xie T, Liu J. Genetic diseases associated with heterotrimeric G proteins. Trends Pharmacol Sci 2006; 27(5): 5260–266.
Reference Link - Eisenhardt A, Siffert W, Rosskopf D, Musch M, Mosters M, Roggenbuck U et al. Association study of the G-protein beta3 subunit C825Tpolymorphism with disease progression in patients with bladder cancer. World J Urol 2005; 23(4): 279-86.
Reference Link - Sheu SY, Handke S, Bröcker-Preuss M, Görges R, Frey UH, Ensinger C et al. The C allele of the GNB3 C825T polymorphism of the G protein beta3-subunit is associated with an increased risk for the development of oncocytic thyroid tumours. J Pathol 2007; 211(1): 60-6.
Reference Link - Lehnerdt GF, Franz P, Bankfalvi A, Grehl S, Jahnke K, Lang S et al. Association study of the G-protein beta3 subunit C825T polymorphism with disease progression an overall survival in patients with head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008; 17(11): 3203-7.
Reference Link - Clar H, Langsenlehner U, Krippl P, Renner W, Leithner A, Gruber G et al. A polymorphism in the G protein beta3-subunit gene is associated with bone metastasis risk in breast cancer patients. Breast Cancer Res Treat 2008; 111(3): 449-52.
Reference Link - Sambrano GR, Chandy G, Choi S, Decamp D, Hsueh R, Lin KM et al. Unravelling the signal-transduction network in B lymphocytes. Nature 2002; 420(6916): 708-10.
Reference Link - Sei Y, Ren-Patterson R, Li Z, Tunbridge EM, Egan MF, Kolachana BS et al. Neuregulin1-induced cell migration is impaired in schizophrenia: association with neuregulin1 and catechol-o-methyltransferase gene polymorphisms. Mol Psychiatry 2007; 12(10): 946–957.
Reference Link - Sawa A, Wiegand GW, Cooper J, Margolis RL, Sharp AH, Lawler JF Jr et al. Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nat Med 1999; 5(10): 1194-8.
Reference Link - Sawa A, Nagata E, Sutcliffe S, Dulloor P, Cascio MB, Ozeki Y et al. Huntingtin is cleaved by caspases in the cytoplasm and translocated to the nucleus via perinuclear sites in Huntington’s disease patient lymphoblasts. Neurobiol Di 2005; 20(2): 267-74.
Reference Link - Nagata E, Sawa A, Ross CA, Snyder SH. Autophagosome-like vacuole formation in Huntington’s disease lymphoblasts. Neuroreport 2004; 15(8): 1325-8
Reference Link - Sun Z, Runne C, Tang X, Lin F, Chen S. The Gβ3 splice variant associated with the C825T gene polymorphism is an unstable and functionally inactive protein. Cell Signal. 2012 Dec; 24(12): 2349-59.
Reference Link - Saini DK, Karunarathne WK, Angaswamy N, Saini D, Cho JH, Kalyanaraman V et al. Regulation of Golgi structure and secretion by receptor-induced G protein bg complex translocation. Proc Natl Acad Sci USA 2010; 107(25): 11417-22.
Reference Link - Rehm A, Ploegh HL. Assembly and intracellular targeting of the betagamma subunits of heterotrimeric G proteins. J Cell Biol 1997; 137(2): 305-317.
Reference Link - Werry TD, Wilkinson GF, Willars GB. Mechanisms of cross-talk between G-protein-coupled receptors resulting in enhanced release of intracellular Ca2+. Biochem. J 2003; 74(Pt 2): 281-96.
Reference Link - Chan AS, Wong YH. Gbetagamma signaling and Ca2+ mobilization co-operate synergistically in a Sos and Rac-dependent manner in the activation of JNK by Gq-coupled receptors. Cell Signal 2004; 16(7): 823-36.
Reference Link - Kong KC, Billington CK, Gandhi U, Panettieri RA Jr, Penn RB. Cooperative mitogenic signaling by G protein-coupled receptors and growth factors is dependent on G(q/11). FASEB J 2006; 20(9): 1558-60.
Reference Link - Price DJ, Grove JR, Calvo V, Avruch J, Bierer BE. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science 1992; 257(5072): 973–977.
Reference Link - Galbaugh T, Cerrito MG, Jose CC, Cutler ML. EGF induced activation of AKT results in mTOR-dependent p70S6 kinase phosphorylation and inhibition of HC11 cell lactogenic differentiation. BMC Cell Biol 2006; (7): 34.
Reference Link - Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in Foxo’s road. Sci STKE 2003; 2003(172): RE5.
- Maruko T, Nakahara T, Sakamoto K, Saito M, Sugimoto N, Takuwa Y et al. Involvement of the bg subunits of G proteins in the cAMP response induced by stimulation of the histamine H1 receptor. Naunyn Schmiedebergs Arch Pharmacol 2005; 372(2): 153-9.
Reference Link - Gill A, Hammes SR. Gbg signaling reduces intracellular cAMP to promote meiotic progression in mouse oocytes. Steroids 2007; 72(2): 117-23.
Reference Link - Dumaz N, Marais R. Protein kinase A blocks Raf-1 activity by stimulating14-3-3 binding and blocking Raf-1 interaction with Ras. J Biol Chem 2003; 278(32): 29819-23.
Reference Link - Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR et al. Microtubules and actin microfilaments regulate lipid raft/caveolae localisation of adenylyl cyclase signaling components. J Biol Chem 2006; 281(36): 26391-9.
Reference Link - Shajahan AN, Tiruppathi C, Smrcka AV, Malik AB, Minshall RD. Gbg activation of Src induces caveolae-mediated endocytosis in endothelial cells. J Biol Chem 2004; 279(46): 48055-62.
Reference Link - Van den Heuvel AP, Schulze A, Burgering BM. Direct control of caveolin-1 expression by Foxo transcription factors. Biochem J 2005; 385(Pt 3): 795-802.
- Yamamoto M, Okumura S, Oka N, Schwencke C, Ishikawa Y. Downregulation of caveolin expression by cAMP signal. Life Sci. 1999; 64(15): 1349-57.
Reference Link - Gao X, Lowry PR, Zhou X, Depry C, Wei Z, Wong GW et al. PI3K/AKT signaling requires spatial compartmentalization in plasma membrane microdomains. Proc Natl Acad Sci U S A 2011; 108(35): 14509-14.
Reference Link - Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003; 112(4): 453-65.
Reference Link - Stahlhut M, van Deurs B. Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol Biol Cell 2000; 11(1): 325–337.
Reference Link - Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y et al. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell 2003; 114(2): 215-27.
Reference Link - Sasaki AT, Janetopoulos C, Lee S, Charest PG, Takeda K, Sundheimer LW et al. G protein-independent Ras/PI3K/F-actin circuit regulates basic cell motility. J Cell Biol. 2007; 178(2): 185-91.
Reference Link - Bulloj A, Duan W, Finnemann SC. PI 3-kinase independent role for AKT in F-actin regulation during outer segment phagocytosis by RPE cells. Exp Eye Res 2013; 113(9-18).
Reference Link - Qian Y, Corum L, Meng Q, Blenis J, Zheng JZ, Shi X et al. PI3K induced actin filament remodeling through Akt and p70S6K1: implication of essential role in cell migration. Am J Physiol Cell Physiol. 2004; 286(1): C153-63.
Reference Link - Urra H, Torres VA, Ortiz RJ, Lobos L, Díaz MI, Díaz N et al. Caveolin-1-enhanced motility and focal adhesion turnover require tyrosine-14 but not accumulation to the rear in metastatic cancer cells. PLoS One 2012; 7(4): e33085.
Reference Link - Zhou HY, Wong AS. Activation of p70S6K induces expression of matrix metalloproteinase 9 associated with hepatocyte growth factor-mediated invasion in human ovarian cancer cells. Endocrinology 2006; 147(5): 2557-66.
Reference Link - Storz P, Döppler H, Copland JA, Simpson KJ, Toker A. Foxo3a promotes tumorcell invasion through the induction of matrix metalloproteinases. Mol Cell Biol 2009; 29(18): 4906-17.
Reference Link - Rybkin II, Cross ME, McReynolds EM, Lin RZ, Ballou LM. alpha(1A) adrenergic receptor induces eukaryotic initiation factor 4E-binding protein 1 phosphorylation via a Ca(2+)-dependent pathway independent of phosphatidylinositol 3-kinase/AKT. J Biol Chem 2000; 275(8): 5460-5.
Reference Link - Conus NM, Hemmings BA, Pearson RB. Differential regulation by calcium reveals distinct signaling requirements for the activation of AKT and p70S6k. J Biol Chem 1998; 273(8): 4776-82.
Reference Link - Cohen JD, Gard JM, Nagle RB, Dietrich JD, Monks TJ, Lau SS. ERK crosstalks with 4EBP1 to activate cyclin D1 translation during quinol-thioether-induced tuberous sclerosis renal cell carcinoma. Toxicol. Sci. 2011; 124(1): 75-87.
Reference Link - Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates Gbg-mediated signaling. J Biol Chem. 1994; 269(8): 6193-7.
- Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279(5350): 509-14.
Reference Link - Masiero L, Lapidos KA, Ambudkar I, Kohn EC. Regulation of the RhoA pathway in human endothelial cell spreading on type IV collagen: role of calcium influx. J Cell Sci 1995; 112(Pt 19): 3205-13.
- Sun CX, Downey GP, Zhu F, Koh AL, Thang H, Glogauer M. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood 2004; 104(12): 3758-65.
Reference Link - Thomas S, Overdevest JB, Nitz MD, Williams PD, Owens CR, Sanchez-Carbayo M et al. Src and caveolin-1 reciprocally regulate metastasis via a common downstream signaling pathway in bladder cancer. Cancer Res 2011; 71(3): 832-41.
Reference Link - Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci 2009; 1(14): 2386-99.
Reference Link - Ren B, Deng Y, Mukhopadhyay A, Lanahan AA, Zhuang ZW, Moodie KL et al. ERK1/2-AKT1 crosstalk regulates arteriogenesis in mice and zebrafish. J Clin Invest 2010; 120(4): 1217-28.
Reference Link - Kageyama K, Ihara Y, Goto S, Urata Y, Toda G, Yano K et al. Overexpression of calreticulin modulates protein kinase B/AKT signaling to promote apoptosis during cardiac differentiation of cardiomyoblast H9c2 cells. J Biol Chem 2002; 277(22): 19255-64.
Reference Link - Yasuoka C, Ihara Y, Ikeda S, Miyahara Y, Kondo T, Kohno S. Antiapoptotic activity of AKT is down-regulated by Ca2+ in myocardiac H9c2 cells. Evidence of Ca(2+)-dependent regulation of protein phosphatase 2Ac. J Biol Chem 2004; 279(49): 51182-92.
Reference Link - Ellis IR, Schor AM, Schor SL. EGF AND TGF-alpha mitogenic activities are mediated by the EGF receptor via distinct matrix-dependent mechanisms. Exp Cell Res 2007; 313(4): 732-41.
Reference Link - Thomas CC, Deak M, Alessi DR, van Aalten DM. High-resolution structure of the pleckstrin homology domain of protein kinase b/AKT bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr Biol 2002; 12(14): 1256–1262.
Reference Link - Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker AAKT blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell 2005; 20(4): 539–550.
Reference Link - Liu H, Radisky DC, Nelson CM, Zhang H, Fata JE, Roth RA et al. Mechanism of AKT1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc Natl Acad Sci USA 2006; 103(11): 4134–4139.
Reference Link - Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N et al. Distinct roles of AKT1 and AKT2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol 2005; 171(6): 1023–1034.
Reference Link - Ellis IR, Jones SJ, Lindsay Y, Ohe G, Schor AM, Schor SL et al. Migration Stimulating Factor (MSF) promotes fibroblast migration by inhibiting AKT. Cell Signal 2010; 22(11): 1655-9.
Reference Link - Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S et al. Foxo3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 2007; 6(6): 472-83.
Reference Link - Kim BW, Choi M, Kim YS, Park H, Lee HR, Yun CO et al. Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin. Cell Signal 2008; 20(4): 714-25.
Reference Link - Du M, Shen QW, Zhu MJ, Ford SP. Leucine stimulates mammalian target of rapamycin signaling in C2C12 myoblasts in part through inhibition of adenosine monophosphate-activated protein kinase. J Anim Sci 2007; 85(4): 919-27.
Reference Link - Beugnet A, Wang X, Proud CG. Target of rapamycin (TOR)-signaling and RAIP motifs play distinct roles in the mammalian TOR-dependent phosphorylation of initiation factor 4E-binding protein 1. J Biol Chem 2003; 278(42): 40717-22.
Reference Link - Iijima Y, Laser M, Shiraishi H, Willey CD, Sundaravadivel B, Xu L et al. c-Raf/MEK/ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells. J Biol Chem 2002; 277(25): 23065-75.
Reference Link - Gu M, Lynch J., Brecher P. Nitric oxide increases p21(Waf1/Cip1) expression by a cGMP-dependent pathway that includes activation of extracellular signal-regulated kinase and p70(S6k). J Biol Chem 2002; 275(15): 11389–11396.
Reference Link - Whelan MC, Senger DR. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J Biol Chem 2003; 278(1): 327–334.
Reference Link - Ip CKM, Cheung ANY, Ngan HYS, Wong AST. p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene 2011; 30(21): 2420-32.
Reference Link - Beardsley A, Fang K, Mertz H, Castranova V, Friend S, Liu J. Loss of caveolin-1 polarity impedes endothelial cell polarization and directional movement. J Biol Chem. 2005; 280(5): 3541-7.
Reference Link - Santilman V, Baran J, Anand-Apte B, Evans RM, Parat MO. Caveolin-1 polarization in transmigrating endothelial cells requires binding to intermediate filaments. Angiogenesis 2007; 10(4): 297-305.
Reference Link - Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 2008; 48:359-91.
Reference Link - Waschke J, Curry FE, Adamson RH, Drenckhahn D. Regulation of actin dynamics is critical for endothelial barrier functions. Am J Physiol Heart Circ Physiol 2005; 288(3): H1296-305.
Reference Link - Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K et al. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res 2003; 92(4): 411-8.
Reference Link - Emamghoreishi M, Schlichter L, Li PP, Parikh S, Sen J, Kamble A et al. High intracellular calcium concentrations in transformed lymphoblasts from subjects with bipolar I disorder. Am J Psychiatry 1997; 154(7): 976-82.
- Newton R, Cambridge L, Hart LA, Stevens DA, Lindsay MA, Barnes PJ. The MAP kinase inhibitors, PD098059, UO126 and SB203580, inhibit IL-1beta-dependent PGE(2) release via mechanistically distinct processes. Br J Pharmacol 2000; 130(6): 1353-61.
Reference Link - Dang VC, Napier IA, Christie MJ. Two distinct mechanisms mediate acute m-opioid receptor desensitization in native neurons. J Neurosci 2009; 29(10): 3322-7.
Reference Link